Extrapontine central myelinolysis with extrapyramidal symptoms in a 14-year-old boy with COVID-19 disease-related PIMS-TS
Authors:
K. Španělová 1*; P. Mužlayová 1*; O. Horák 1; J. Šenkyřík 2; M. Malá 3; J. Klučka 4; H. Ošlejšková 1; P. Danhofer 1
Authors place of work:
These authors contributed equally to the paper as first authors.
*; Department of Pediatric Neurology, Faculty of Medicine of Masaryk University, University Hospital Brno, Czech Republic
1; Department of Pediatric Radiology, Faculty of Medicine of Masaryk University, University Hospital Brno, Czech Republic
2; Department of Pediatric Infectious Diseases, Faculty of Medicine of Masaryk University, University Hospital Brno, Czech Republic
3; Department of Pediatric Anesthesiology and Intensive Care Medicine, Faculty of Medicine of Masaryk University, University Hospital Brno, Czech Republic
4
Published in the journal:
Cesk Slov Neurol N 2022; 85(6): 503-505
Category:
Dopis redakci
doi:
https://doi.org/10.48095/cccsnn2022503
Dear Editor,
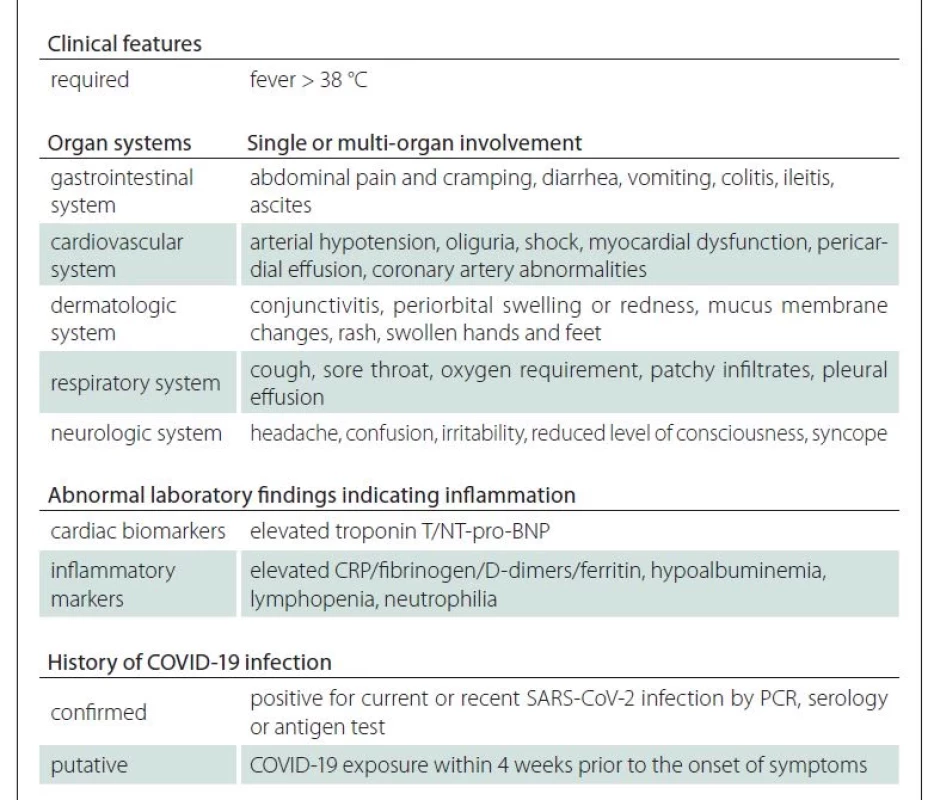
Coronavirus disease 2019 (COVID-19) has spread throughout the world following an outbreak in China in 2019. Severe acute respiratory syndrome coronavirus 2 (SARS- -CoV-2) primarily targets the respiratory system. In symptomatic patients, the most common symptoms are fever, cough, and headache. Severe forms can present with pneumonia, acute respiratory distress syndrome, acute cardiac dysfunction, and multiorgan failure [1]. Most reports have indicated that adolescents and children follow a milder clinical course [2]. Nevertheless, recent reports alerted the medical community to a pediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS) [3]. Diagnostic criteria and management of PIMS-TS are already relatively well defined (Tab. 1) [3].
To further define PIMS-TS and to better elucidate its pathophysiology, we present an unusual case report of a patient who developed central extrapontine myelinolysis (EPM) after COVID-19 infection with PIMS-TS.
A previously healthy 14-year-old boy presented with fever (39 °C) unresponsive to antipyretic treatment, sore throat, abdominal pain, vomiting, and palmoplantar rash. Despite the introduced antibiotic therapy, his condition quickly worsened. He was admitted via the emergency room due to impaired consciousness and ventricular fibrillation that required cardiopulmonary resuscitation, electrical cardioversion, and subsequent artificial lung ventilation. The septic condition was complicated by the onset of disseminated intravascular coagulopathy and temporary circulatory instability with the need for catecholamines, even though the acute echocardiogram was normal without signs of cardiomyopathy, no intracardial thrombi were found and the patient had no risk of thromboembolism. It was also necessary to correct a mild transient electrolyte imbalance. An X-ray of the lungs verified a left-sided lobar pneumonia. A double combination of antibiotics (meropenem + vancomycin) was added to the medication. The initial SARS-CoV-2 PCR test was repeatedly negative over a period of 5 days. The examination of blood cultures, CSF, and bronchoalveolar lavage did not identify any infectious agent and the therapy was augmented by an antiviral drug (aciclovir). An extensive autoimmune serologic screening was performed (including onconeural antibodies and neural membrane antibodies) with a negative result. In the CSF, positivity of immunoglobulin G (IgG) and M (IgM) antibodies against Borrelia burgdorferi sensu lato was found (the results were confirmed by Western Blot confirmatory test; intrathecal synthesis antibody index AI-IgG was 2 [positivity from 1.4], AI-IgM negative) and serology test confirmed positive IgG and IgM antibodies against enteroviruses. Although the diagnosis of neuroborreliosis (NB) was unlikely (no lymphocytic pleocytosis in the CSF and no typical clinical presentation of the NB), the antibiotic therapy with meropenem was added to the medication for 14 days. The control lumbar puncture was not indicated. No convulsions were observed in the patient, and repeated EEG was normal without any epileptiform activity. Due to psychomotor agitation and conjugated eye deviation with vertical nystagmus which developed after early extubation, we performed acute brain MRI. It showed bilateral hyperintensities in the putamen and globus pallidus, most likely corresponding with EPM (Fig. 1 – A1, A2). Differential diagnosis of such basal ganglia hyperintensities in MRI includes hypoglycemia, hypoxic ischemic encephalopathy or acute exposure to carbon monoxide poisoning. Ischemic disorder was excluded by the absence of diffusion restriction in diffusion weighted imaging. Hypoglycemia could be ruled out by blood sugar level monitoring, and hypoxia and poisoning via history.
FLAIR – fluid attenuated inversion recovery
Obr. 1. Akutní MR mozku: (A1) FLAIR a (A2) T2 vážený sken v axiální rovině: symetrické hyperintenzity jak v nucleus caudatus, tak v putamenu.
Kontrolní MR mozku: (B1) FLAIR a (B2) T2 vážený sken v axiální rovině: změny signálu a oboustranná atrofie putamen s cystickou
transformací primárně vlevo, hyperintenzity v nucleus caudatus oboustranně.
FLAIR – fluid attenuated inversion recovery

The control brain MRI with MRA 12 days later was stationary.
Three weeks after his admission to the hospital, high titers of antibodies were detected in blood (anti SARS-CoV-2 S was 465.9 cut-off index).
Further clinical presentation was dominated by hypokinetic-rigid syndrome – dysarthria, dysphagia, rigidity, bradykinesia, tremor with mild central quadriparesis, saccadic eye movement, and intermittent horizontal nystagmus. One month after the clinical onset, the MRI showed atrophy and cystoid transformation in the bilateral basal ganglia (Fig. 1) – B1, B2. The clinical condition improved slowly following the introduction of physiotherapy. Six months after the clinical onset, the residual dysarthria, reflexological predominance on the left-sided extremities and upper extremities dystaxia was present. The psychological examination indicated memory impairment and impairment in auditory attention.
The condition was evaluated as PIMS-TS. Initial co-occurrence with enterovirus or other viral infection cannot be ruled out.
Central EPM is an osmotic demyelinating syndrome (ODS) and mainly affects the basal ganglia, thalamus, and subcortical white matter [4]. In a large Swedish study with 83 patients with ODS, only 4% had an isolated image of EPM [5]. Only two case studies of patients with ODS related to COVID-19 have been published [6,7]. The course of COVID-19 is mostly mild in children. However, more studies have emerged indicating a complicated course in children, especially with the development of PIMS-TS [8,9]. A large study with 58 children with PIMS-TS was recently published in which the clinical manifestation was: fever (in all cases), abdominal pain (53%), rash (52%), nausea and/or vomiting (50%), and conjunctivitis (45%); 22% met the diagnostic criteria for Kawasaki syndrome and 14% had coronary artery dilation or aneurysm. No neurological clinical manifestations or complications have been reported [9].
The PIMS-TS in our patient was complicated by the development of neurological symptoms with EPM confirmed by brain MRI. However, a mild electrolyte imbalance was detected during the course, first hyponatremia and hyperphosphatemia, then hypernatremia and hypophosphatemia later in the hospital. The correction of the electrolyte imbalance was very slow according to valid guidelines; therefore, it is unlikely to be the cause of EPM. Mild hepatopathy and nephropathy were found according to laboratory tests, but there was a rapid adjustment of renal and liver parameters. Other causes of EPM such as hypokalemia, severe hepatopathy, or other symptoms were not identified during a detailed examination. EPM can manifest in diverse presentations, such as spastic paraparesis, mutism, catatonia, myoclonic jerks, and Parkinsonian features with choreoathetosis or dystonia [10]. Rigidity, hypomimia, and cognitive impairment dominated the clinical neurological picture of our patient.
It is generally believed that COVID-19 infection in children usually has a benign course. However, we are more likely to encounter diseases that we have rarely seen before that share a common denominator of past COVID-19 infections. Further studies will certainly be needed to address the pathophysiological basis of this infection to ensure its optimal management.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in this work.
Pavlína Danhofer, MD, PhD
Department of Pediatric Neurology
Faculty of Medicine of Masaryk
University
University Hospital Brno
Černopolní 9
613 00 Brno
Czech Republic
e-mail: danhofer.pavlina@fnbrno.cz
Accepted for review: 9. 6. 2022
Accepted for print: 1. 12. 2022
Zdroje
1. Hanafi R, Roger PA, Kuchcinski G et al. COVID-19 Neurologic complications with CNS vasculitis-like pattern. Am J Neuroradiol 2020; 41 (8): 1384–1387. doi: 10.3174/ajnr.A6651.
2. Dong Y, Mo X, Hu Y et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020; 145 (6): e20200702. doi: 10.1542/peds.2020-0702.
3. Regev T, Antebi M, Eytan D et al. Pediatric Inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 infection. Pediatr Infect Dis J 2020; 39 (8): e206–e207. doi: 10.1097/INF.0000000000002 804.
4. Slanina M, Žižka J, Klzo L et al. Osmotický demyelinizační syndrom – diagnostika magnetickou rezonancí: kazuistika. Cesk Slov Neurol N 2007; 70/103 (3): 322–327.
5. Aegisdottir H, Cooray C, Wirdefeldt K et al. Incidence of osmotic demyelination syndrome in Sweden: a nationwide study. Acta Neurol Scand 2019; 140 (5): 342–349. doi: 10.1111/ane.13150.
6. Abd Ur Rehman M, Abdulrahman AF, Zainab A et al. Hyponatremia and extrapontine myelinolysis in a patient with COVID-19: a case report. Clin Case Rep 2021; 9 (7): e04463. doi: 10.1002/ccr3.4463.
7. Jensen-Kondering U, Neumann A, Margraf NG et al. Cerebral imaging in patients with COVID-19 and neurological symptoms: first experience from two University Hospitals in Northern Germany. Rofo 2021; 193 (6): 667–671. doi: 10.1055/a-1265-7209.
8. Regev T, Antebi M, Eytan D et al. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 infection. Pediatr Infect Dis J 2020; 39 (8): e206–e207. doi: 10.1097/INF.0000000000002804.
9. Yasuhara J, Watanabe K, Takagi H et al. COVID-19 and multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Pulmonol 2021; 56 (5): 837–848. doi: 10.1002/ppul.25245.
10. Halim SA, Mohd Amin NA. Treatment response in osmotic demyelination syndrome presenting as severe parkinsonism, ptosis and gaze palsy. BMJ Case Rep 2018: bcr2018225751. doi: 10.1136/bcr-2018-225751.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2022 Číslo 6
Nejčtenější v tomto čísle
- Guidelines for developmental dysphasia – version 2022
- Validation study and introduction of the new TEPO sentence comprehension test for children aged 3–8 years
- New pharmacological options in the treatment of Alzheimer‘s disease
- Limb girdle muscular dystrophies
