A Heart Rate Turbulence Study to Assess Cardiac Autonomic Function in Migraineurs
Turbulence srdeční frekvence v posouzení kardiální autonomní funkce u migreniků
Studie autonomní funkce u pacientů s migrénou nám dávají protichůdné výsledky. Turbulence srdeční frekvence (HRT) je novou metodou používanou k hodnocení kardiální autonomní funkce. Zhoršená HRT odráží kardiální autonomní dysfunkci. Cílem této studie bylo určit validaci HRT jakožto testu autonomní funkce u migreniků a srovnat ji s normální populací. Studie zahrnovala 44 pacientů s migrénou a 50 zdravých jedinců. Zaznamenávali jsme ambulantní 24hodinové elektrokardiogramy a vypočítali jsme parametry HRT, nástup turbulence (TO) a strmost turbulence (TS). HRT nemohla být vypočtena u 16 migreniků a 24 kontrol, protože tyto výpočty nelze provádět u subjektů, u kterých v záznamech Holteru chybí komorové extrasystoly. Tito pacienti byli ze statistických analýz vyloučeni. HRT jsme vypočítali u 28 migreniků (průměrný věk 47,4 ± 10,1 roků, 20 žen) a 28 kontrol (průměrný věk 47,5 ± 11, 6 roků, 20 žen). Ve dvou parametrech HRT nebyly zjištěny žádné významné rozdíly; TO a TS mezi migreniky a kontrolami (TO migreniků: -0,65 ± 3,84%, TO kontrol: –0,61 ± 4,42%, p = 0,38; TS migreniků: 9,34 ± ± 7,50 ms/RR, TS kontrol: 8,82 ± 6,34 ms /RR, p = 0,40). Zdá se, že parametry HRT, které určují kardiální autonomní dysfunkci, se u pacientů s migrénou nezměnily.
Klíčová slova:
kardiální autonomní funkce – turbulence srdeční frekvence – migréna
Authors:
A. Avsar 1; M. Yaman 2; C. Kilit 1; M. Melek 1; H. Saglam 1; E. Onrat 1
Authors place of work:
Department of Cardiology, School of Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey
1; Department of Neurology, School of Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey
2
Published in the journal:
Cesk Slov Neurol N 2007; 70/103(2): 174-177
Category:
Původní práce
Summary
Studies of autonomic nervous system function in patients with migraine have shown conflicting results. Heart rate turbulence (HRT) is a new method to assess cardiac autonomic function. HRT impairment reflects cardiac autonomic dysfunction. The aim of the present study was to determine validation of HRT as an autonomic function test in patients with migraine in comparison with the normal population. Forty-four patients with migraine and fifty healthy subjects were included to the study. Twenty-four hours ambulatory electrocardiograms were recorded. HRT parameters, turbulence onset (TO) and turbulence slope (TS) were calculated. In 16 migraineurs and 22 control subjects we did not able to calculate HRT because HRT calculation is impossible in subjects who do not have any ventricular premature beat (VPB) in their Holter recordings. These subjects excluded from statistical analyses. HRT were calculated in 28 migraineurs (mean age 47.4 ± 10.1 years, 20 women) and 28 control subjects (mean age 47.5 ± 11.6 years, 20 women). There were no significant differences in two HRT parameters; TO and TS between migraineurs and control subjects (TO migraineur: -0.65 ± 3.84%, TO control: -0.61 ± 4.42%, p=0.38; TS migraineur: 9.34 ± 7.50 ms/RR, TS control: 8.82 ± 6.34 ms/RR, p=0.40, respectively). HRT parameters, which determine the cardiac autonomic dysfunction, did not seem to be altered in patients with migraine.
Key words:
cardiac autonomic function, heart rate turbulence, migraine
Introduction
Migraine headaches are generally thought to be associated with arterial vasomotor and autonomic nervous system abnormality [1]. The clinical symptoms of migraine point to autonomic disturbances, especially to disrupted regulation of the circulatory system and autonomic balance. The autonomic activities in patients with migraine have been studied previously and these studies examining autonomic activity in migraineurs have given conflicting results. The most common finding is sympathetic hypofunction but sympathetic hyperfunction or parasympathetic dysfunctions have also been reported [2-4]. However it is not known definitely, whether the autonomic dysfunction is a result or a reason of migraine [5].
Heart rate turbulence (HRT) is a new diagnostic method to assess autonomic and reflex modulations of cardiac function like heart rate variability (HRV) and baroreflex sensitivity (BRS) [6]. HRT impairment reflects cardiac autonomic dysfunction, in particular impaired BRS and reduced parasympathetic activity [6,7]. Therefore, HRT can be used as a noninvasive measure of cardiac autonomic dysfunction [8]. The exact physiological mechanisms of HRT are not fully identified, but it is believed that HRT is caused baroreflex mechanism [9].
In migraineurs, studies investigating cardiac autonomic function are inconclusive with reports of both hyperfunction and hypofunction of the sympathetic and parasympathetic nervous systems. However, HRT has not been studied as a cardiac autonomic function test in migraineurs yet. The aim of the present study was to evaluate cardiac autonomic function in patients with migraine by using HRT parameters.
Material and method
Study populations
Forty four patients (mean age 46.5 ± 11.2 years, thirty women) with migraine who were already being followed by the Department of Neurology participated in this study. The diagnosis of migraine was established according to criteria of Headache Classification Committee of the International Headache Society [10]. The subjects who have non-sinus rhythm, coexisting valvular heart disease, unstable angina, myocardial infarction, heart failure, hyperthyroidism, left ventricular hypertrophy, electrolyte disturbances, cardioactive drugs (especially beta blockers and/or antiarrhythmic drugs) and diabetes mellitus were excluded. A control group was formed by fifty healthy adults of similar ages (mean age 45.3 ± 9.5 years, thirty-two women). All participants’ physical examinations, resting 12-lead electrocardiograms and exercise tests were normal. Routine biochemical and hematological values including fasting blood glucose, blood urea nitrogen, serum electrolytes, thyroid hormones and hemoglobin levels were in normal ranges. All participants (44 migraineurs and 50 control subjects) underwent 24 hours Holter electrocardiogram. In 16 migraineurs and 22 control subjects we did not able to calculate HRT because HRT calculation is impossible in subjects who do not have any ventricular premature beat (VPB) in their Holter recordings. These subjects excluded from statistical analyses. HRT were calculated in 28 migraineurs (mean age 47.4 ± 10.1 years, 20 women) and 28 control subjects (mean age 47.5 ± 11.6 years, 20 women) who had at least one VPB in their Holter recordings.
HRT analysis
Holter recordings were analyzed with Reynolds Medical Pathfinder Software Version V8.255 (Hedford, England). HRT was calculated with HRT! View Version 0.60-0.1 software program. Firstly, abnormal beats and areas of artifact which were accepted as VPB by computer were manually identified and excluded while determining HRT. The HRT parameters were determined according to the original method [11]. Turbulence onset (TO), which is a measure of the early sinus acceleration after a VPB, is expressed as a percentage and is calculated with the following formula: [(RR1+RR2)-(RR-2+RR-1)]/(RR-2+RR-1)x100, where RR1 and RR2 are the first and second sinus RR intervals after the VPB, and RR-1 and RR-2 are the first and the second sinus RR intervals preceding the VPB. Turbulence slope (TS), which is a measure of the late sinus deceleration after a VPB, is obtained as the maximal positive slope among all slopes of a series of regression lines obtained from all sequences of 5 consecutive RR intervals included between the first and the 20th RR interval following the VPB, and expressed as ms/RR. TO was calculated for all VPB’s separately and then averaged, whereas TS was calculated based on an averaged local tachogram. TO and TS were defined as abnormal if the onset was > 0% or the slope was < 2.5 ms/RR, respectively.
Statistical analysis
Statistical analyses were performed with SPSS for Windows version 10.0 (SPSS Inc, Chicago, Illinois). Differences between groups were analyzed by the Mann-Whitney U test and chi-square test, as appropriate. A p value < 0.05 was considered as statistically significant. The data were presented as mean ± SD.
Results
Age distribution and the gender ratio were similar for both groups. HRT onset and slope did not differ between migraineurs and controls (TO migraineur: -0.65 ± 3.84%, TO control: -0.61 ± 4.42%, p=0.38; TS migraineur: 9.34 ± 7.50 ms/RR, TS control: 8.82 ± 6.34 ms/RR, p=0.40, respectively) (tab. 1)
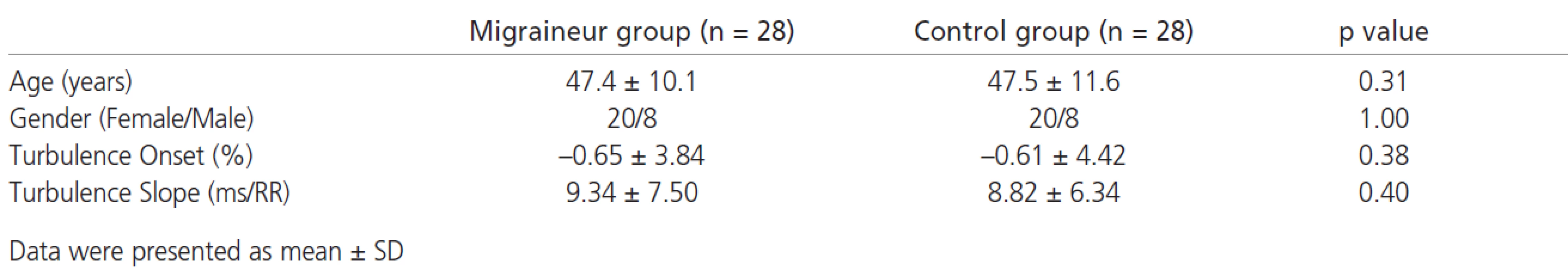
Discussion
As a cardiac autonomic function test, this study is the first HRT study of migraine. In this study, HRT parameters (TO and TS) did not alter in migraineurs.
Previous studies showed that there was an autonomic dysfunction and reduced HRV in patients with migraine, which indicate parasympathetic nervous system hypofunction [5,12-14]. In some studies, sympathetic nervous system dysfunctions have been determined [13,15-16]. It was known that impaired sympathetic activity caused a decrease in arrhythmia threshold.
During migraine attacks, asystole, bradycardia, atrial fibrillation and bundle branch block have been determined. Also, syncope attacks have been seen in these arrhythmia periods. Although it has been speculated that these arrhythmias have been occurred during migraine attacks, there were no clear studies determining the arrhythmias in headache free period [17-19].
The heart is richly innervated by afferent and efferent vagal and sympathetic fibers and is thus susceptible to autonomic influences [20]. So the changes in efferent autonomic traffic to the heart play a critical role in the genesis and outcome of cardiac arrhythmias. Increased sympathetic and decreased vagal tone can interact with all of the electrophysiological mechanisms underlying arrhythmogenesis. The fact that changes in efferent autonomic traffic are largely under baroreceptor control explains why baroreceptor function is correlated with cardiac arrhythmias [21]. HRT, BRS and HRV provide different information about cardiac autonomic function and they predict mortality in heart disease [22]. Moreover, the moderate correlation between BRS and HRV (r= 0.63) suggested that the two measures explore different functions of autonomic control and that they might provide prognostic information of incremental value. Bigger et al. have shown that altered HRV has been determined after myocardial infarction; baroreflex sensitivity and HRT were found as normal in the same group. Bigger et al have shown that there was a weak correlation between baroreflex sensitivity and Holter measures of HRV in myocardial infarction [23]. Also Ortak et al have shown that in the same patient group after myocardial infarction, indices of HRV were increased but HRT parameters were not changed [24]. As a result HRT and HRV reflect different aspects of cardiac autonomic function.
HRT is highly correlated with spontaneous BRS and it may be used instead of BRS [25]. It is proven that HRT also predicts mortality and sudden cardiac death in various cardiac abnormalities like postmyocardial infarction period, after coronary artery by-pass grafting surgery and in chronic heart failure [22,26,27]. In addition, HRT predicts alterations of autonomic cardiac functions in diabetes mellitus and hyperthyroidism [28,29].
In a study evaluating baroreflex functions in patients with migraine, Sanya et al found that baroreflex-mediated cardiovagal responses are reduced in migraine patients [30]. On the other hand, Pogacnik et al and Mikamo et al did not find any deterioration in cardiac autonomic function in patients with migraine by using HRV [13,31]. So, we want to investigate HRT in patients with migraine as unknown arm of cardiac autonomic function and we found that HRT parameters remain normal in patients with migraine. Although Mikamo et al and Pogacnik et al investigated cardiac autonomic function by using HRV, our findings seems to be similar with their results.
Although migraine is associated with cardiac autonomic nervous system abnormalities in previous studies, HRT parameters which determine the cardiac autonomic dysfunction did not seem to be altered in patients with migraine in present study. These results are in contrast to the data of other authors indicating cardiac autonomic dysfunction in migraineurs. It is difficult to explain different results of all studies, involving cardiac autonomic function in migraine. This may be partly due to procedural discrepancies or possibly reveal heterogeneity in cardiac autonomic nervous system in migraineurs [32]. The constitutional and genetic factors may play role in these different results.
The main limitation of our study seems to be the small sample size. The HRT method that we used in the study, could calculate turbulence onset and turbulence slope parameters, approximately in half of patients. HRT parameters should not be calculated in patients who did not have VPB in their Holter recordings.
Consequently, HRT parameters, which determine the cardiac autonomic dysfunction, did not seem to be altered in patients with migraine. Comprehensive cardiac autonomic function studies must be performed in this disease. These findings need to be confirmed with larger studies.
Dr. Alaettin AVSAR,
Karaman Mahallesi, Leylak Caddesi
Manolya Apartmani, No: 13/5
Afyonkarahisar – TURKEY
e-mail: alavsar@hotmail.com, alaettina@yahoo.com
Přijato k recenzi: 20. 6. 2006
Přijato do tisku: 19. 9. 2006
Zdroje
1. Laffitte C, Even C, Henry-Lebras F, de Toffol B, Autret A. Migraine and angina pectoris by coronary artery spasm. Headache 1996; 36: 332-4.
2. Appel S, Kuritzky A, Zahavi I, Zigelman M, Akselrod S. Evidence for instability of the autonomic nervous system in patients with migraine headache. Headache 1992; 32: 10-17.
3. Havanka-Kanniainen H, Tolonen U, Myllyla VV. Autonomic dysfunction in migraine: a survey of 188 patients. Headache 1998; 28: 465-70.
4. Thomsen LL, Iversen HK, Boesen F, Olesen J. Transcranial Doppler and cardiovascular responses during cardiovascular autonomic tests in migraineurs during and outside attacks. Brain 1995; 118: 1319-13.
5. Yakinci C, Mungen B, Er H, Durmaz Y, Karabiber H. Autonomic nervous system function in childhood migraine. Pediatr Int 1999; 41: 529-33.
6. Mrowka R, Persson PB, Theres H, Patzak A. Blunted arterial baroreflex causes "pathological" heart rate turbulence. Am J Physiol Regul Integr Comp Physiol 2000; 279: 1171-5.
7. Marine JE, Watanabe MA, Smith TW, Monahan KM. Effect of atropine on heart rate turbulence. Am J Cardiol 2002; 89: 767-9.
8. Guzik P, Schmidt G. A phenomenon of heart-rate turbulence, its evaluation, and prognostic value. Card Electrophysiol Rev 2002; 6: 256-61.
9. Bauer A, Schmidt G. Heart rate turbulence. J Electrocardiol 2003: 36: 89-93.
10. Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgia, and facial pain. Cephalalgia 1988; 8: 1-96.
11. Malik M, Wichterle D, Schmidt G. Heart rate turbulence. G Ital Cardiol 1999; 29: 65-9.
12. Araki N. Autonomic nervous activity in migraine. Rinsho. Shinkeigaku 1995; 35: 1336-8.
13. Pogacnik T, Sega S, Pecnik B, Kiauta T. Autonomic function testing in patients with migraine. Headache 1993; 33: 545-50.
14. Sliwka U, Harscher S, Diehl RR, van Schayck R, Niesen WD, Weiller C. Spontaneous oscillations in cerebral blood flow velocity give evidence of different autonomic dysfunctions in various types of headache. Headache 2002; 41: 157-63.
15. Peroutka SJ. Migraine: a chronic sympathetic nervous system disorder. Headache 2004; 44: 53-64.
16. Ostertag D, Strittmatter M, Schimrigk K. Autonomic dysfunction in migraine und tension-type headache-pilot study. Schmerz 1998; 12: 25-9.
17. Russell D, Storstein L. Chronic paroxysmal hemicrania: heart rate changes and ECG rhythm disturbances. A computerized analysis of 24 h ambulatory ECG recordings. Cephalalgia 1984; 4: 135-44.
18. Lewis NP, Fraser AG, Taylor A. Syncope while vomiting during migraine attack. Lancet 1988; 13: 400-1.
19. Shuaib A, Klein G, Dear R. Migraine headache and atrial fibrillation. Headache 1987; 27: 252-3.
20. Malliani A, Recordati G, Schwartz PJ. Nervous activity of afferent cardiac sympathetic fibers with atrial and ventricular endings. J Physiol 1973; 229: 457-9.
21. Schwartz PJ, Zipes DP. Autonomic modulation of cardiac arrhythmias. In: Zipes DP, Jalife J (eds). Cardiac Electrophysiology. From Cell to Bedside. Philadelphia: WB Saunders 1995: 300-14.
22. La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet 1998; 351: 478-84.
23. Bigger JT Jr, La Rovere MT, Steinman RC, Fleiss JL, Rottman JN, Rolnitzky LM et al. Comparison of baroreflex sensitivity and heart period variability after myocardial infarction. J Am Coll Cardiol 1989; 14: 1511-8.
24. Ortak J, Weitz G, Wiegand UK, Bode F, Eberhardt F, Katus HA et al. Changes in heart rate, heart rate variability, and heart rate turbulence during evolving reperfused myocardial infarction. Pacing Clin Electrophysiol 2005; 28 (Suppl 1): 227-32.
25. Lin LY, Lai LP, Lin JL, Du CC, Shau WY, Chan HL et al. Tight mechanism correlation between heart rate turbulence and baroreflex sensitivity: sequential autonomic blockade analysis. J Cardiovasc Electrophysiol 2002; 13: 427-31.
26. Koyama J, Watanabe J, Yamada A, Koseki Y, Konno Y, Toda S et al. Evaluation of heart-rate turbulence as a new prognostic marker in patients with chronic heart failure. Circ J 2002; 66: 902-7.
27. Schmidt G, Malik M, Barthell P, Schneider R, Ulm K, Rolnitzky L et al. Heart rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999; 353: 1390-6.
28. Barthel P, Schmidt G, Schneider R, Ulm K, Malik M, Schomig A. Heart rate turbulence in patients with and without autonomic dysfunction. J Am Coll Cardiol 1999; 33 (Suppl): 136A.
29. Osman F, Franklyn JA, Daykin J, Chowdhary S, Holder RL, Sheppard, MC et al. Heart rate variability and turbulence in hyperthyroidism before, during, and after treatment. Am J Cardiol 2004; 94: 465-9.
30. Sanya EO, Brown CM, von Wilmowsky C, Neundorfer B, Hilz MJ. Impairment of parasympathetic baroreflex responses in migraine patients. Acta Neurol Scand 2005; 111: 102-7.
31. Mikamo K, Takeshima T, Takahashi K. Cardiovascular sympathetic hypofunction muscle contraction headache and migraine. Headache 1989; 29: 86-9.
32. Pierangeli P, Parchi P, Barletta G, Chiogna M, Lugaresi E, Cortelli P. Power spectral analysis of heart rate and diastolic blood pressure variability in migraine with and without aura. Cephalalgia 1997; 17: 756-60.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2007 Číslo 2
Nejčtenější v tomto čísle
- Epilepsy and the Sleep-Waking Cycle
- The Current View of the Diagnostics and Therapy of Aphasias
- Complications of the Anterior Cervical Spine Surgery for a Degenerative Disease
- Worsening of Epileptic Seizures and Epilepsies due to Antiepileptic Drugs – is it Possible?
