Free serum triiodothyronine associations with the Mini-Mental State Examination score after experienced acute ischemic stroke
Souvislost mezi hladinou volného trijodtyroninu v séru a skóre Mini-Mental State Examination po prodělané akutní ischemické CMP
Cíl: Cílem této studie bylo určit souvislost mezi hormony produkovanými hypotalamo-hypofyzárně-tyroidní (tyroidní) osou (thyroid axis produced hormones; TAPH) a kognitivním stavem po prodělané akutni ischemické CMP (acute ischemic stroke; AIS). Materiál a metody: U jedinců s AIS z tří různých výzkumných a klinických center byla stanovena hladina TAPH v séru, vč. tyreostimulačního hormonu, volného tyroxinu a volného trijodtyroninu (FT3), a to po přijetí pacienta a před jeho propuštěním. Kognitivní funkce byly hodnoceny během akutní ubakutní fáze AIS pomocí Mini-Mental State Examination (MMSE). Výsledky: Byla získána data týkající se 194 jedinců s AIS v akutní fázi a 89 jedinců s AIS v subakutní fázi. Během akutní fáze AIS byla nezávislou determinantou kognitivního stavu hladina FT3 (R2 = 0,016; p = 0,017). Během subakutní fáze AIS nebyly zjištěny žádné nezávislé asociace mezi změřenými hladinami hormonů v séru a MMSE. Závěr: Hladina FT3 v séru změřená při přijetí pacienta s AIS v akutní fázi může predikovat jeho kognitivní stav hodnocený pomocí MMSE.
Klíčová slova:
ischemická cévní mozková příhoda – tyreostimulační hormon – volný trijodotyronin – volný tyroxin – kognitivní stav
Authors:
S. Taroza; J. Burkauskas; A. Podlipskyté; N. Mickuviené
Authors‘ workplace:
Neuroscience Institute, Lithuanian University of Health Sciences, Kaunas, Lithuania
Published in:
Cesk Slov Neurol N 2023; 86(1): 74-81
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn202374
Overview
Aim: The aim of this study was to determine association between hypothalamic-hypophysis-thyroid axis (thyroid axis) produced hormones (TAPH) and cognitive performance after experienced acute ischemic stroke (AIS). Materials and methods: Individuals with AIS from three different research and clinical centers were evaluated for serum TAPH levels, including thyroid stimulating hormone, free thyroxin, and free triiodothyronine (FT3) during admission and before discharge. Cognitive outcomes were evaluated using the Mini-Mental State Examination (MMSE) test during an acute and a subacute AIS periods. Results: Data were available for 194 and 89 individuals during an acute and a subacute AIS periods, respectively. FT3 (R2 = 0.016; P = 0.017) was an independent determinant of cognitive performance during an acute AIS period. No independent associations were established between measured hormone serum levels and MMSE estimate during subacute AIS period. Conclusion: FT3 serum levels on admission could predict cognitive performance assessed by the MMSE during an acute AIS period.
Keywords:
ischemic stroke – thyroid stimulating hormone – free triiodothyronine – free thyroxine – cognitive performance
Introduction
Cognitive impairment after stroke (CIAS) is one of the most commonly determined post-stroke consequences experienced in approximately one in two stroke survivors in hospital-based studies [1]. The course of CIAS is not uniform [2] and further decline in cognitive functions is inherent, and faster than in the normal aging population [3]. The burden of CIAS is associated with its negative outcomes, such as reduced activity and participation in life situations [4], increased rate of institutionalization [5], depression [6], elevated risk of ischemic stroke [7], decreased effectiveness of rehabilitation [8], and even death [9,10]. Taking into account these undesirable consequences of CIAS, all stroke survivors should be screened for cognitive deterioration early after stroke for further, more comprehensive evaluation and possible intervention for better outcomes of CIAS [4]. Many neuropsychological instruments have been created for cognitive function assessment, such as the most frequently used Mini-Mental State Examination (MMSE) [11]. The shortcomings of this and other neuropsychological instruments include their dependency on individual’s age and education, and the requirement for special training to perform them. To overcome these limitations, there is a constant search for other markers assisting in CIAS diagnosis and prognosis [12], including molecular biomarkers [13]. Attractive molecular biomarkers are those which can be measured in serum, are cost effective, and distinguish the condition with high sensitivity and specificity [14]. Unfortunately, due to the complexity of stroke itself and uniqueness of each individual biological molecular communication network and many confounders affecting it, the existence of one ideal molecular biomarker is generally questioned [14]. Nevertheless, the research on molecular biomarkers for combining them into panels for improved differentiation is ongoing [15]. One of the most promising molecular biomarkers depends on hypothalamic-pituitary-thyroid axis (thyroid axis) produced hormones (TAPH), such as thyroid-stimulating hormone (TSH) and thyroid hormones (TH), whose principal representatives are prohormone thyroxine and active hormone triiodothyronine (T3), often tested to determine thyroid function in everyday clinical practice. In addition, this study was also prompted by the established TH serum links in individuals with coronary artery disease [16,17].
Stroke, as a catastrophic brain disaster, is not limited to the cerebral tissue, but induces systemic pathophysiological responses including the neuroendocrine thyroid axis response [18]. Functional alterations of this axis during an acute stroke period usually manifest with lowered T3 levels [19] and increased risk of acquired hypothyroidism in a later period of ischemic stroke [20]. Lowered T3 serum levels after stroke are associated with worse functional outcomes, including global and basic activities of daily living [19], symptoms of depression [21], and mortality [22].
Thyroid axis produced hormones at the right time, in the right place, and at the right levels are an integral part for normal brain development and function, encompassing among others cognition and behaviour, during the whole life course [23,24]. Cognitive dysfunction, although sometimes with conflicting results, was determined in thyroid disorders ranging from overt to subclinical hyper- or hypoactive thyroid and even in individuals with normal thyroid function [25]. At least three studies investigated links between a thyroid profile after experienced ischemic stroke and CIAS, with inconsistent results [26–28].
We focused this observational study on establishing the associations between TAPH, i.e., TSH, free thyroxine (FT4) and free T3 (FT3) and post-ischemic stroke cognitive performance estimates in individuals with acute ischemic stroke (AIS).
Materials and methods
Study protocol
The details of the protocol are described elsewhere [29,30].
Study population
Neurologists involved in the study invited all individuals with diagnosed AIS admitted to the departments of neurology at Klaipeda University Hospital and the Hospital of the Lithuanian University of Health Sciences Kauno Klinikos, Lithuania over a 6-month period during the years 2013 and 2014, respectively, and to Klaipeda Seamen’s Hospital, Lithuania over a 12-month period starting in the fall of 2016 to participate in the study. We included individuals aged 18 to 80 years in the study if presenting within the first 48 h of AIS onset. We defined AIS in accordance with the World Health Organization criteria [31] confirming its diagnosis with a brain CT scan. Possible AIS mimics (postictal palsy, migraine, tumor, demyelinating diseases) were excluded on the basis of brain MRI results.
We applied the following exclusion criteria for all individuals: known thyroid disease, ongoing intake of thyroid affecting drugs (thyroxin, amiodarone, steroids, heparin, carbamazepine, iodinated contrast), serious renal and/or liver insufficiency, cancer, infection, TAPH profile not in accordance with biochemical euthyroidism, low-T3 syndrome, subclinical hypoactive or hyperactive thyroid. A total of 612 individuals presenting with AIS during the study enrolment period were invited to participate in the study.
Study design
On admission, a neurologist involved in the study collected baseline data: age, sex, neurological impairment according to the National Institutes of Health Stroke Scale (NIHSS), disability before stroke according to the modified Rankin Scale (mRS), application of intravenous thrombolysis for index stroke treatment or not, arterial blood pressure, use of anticoagulants or antiplatelets before index stroke or not, and cardiovascular risk factors history: arterial hypertension, atrial fibrillation (AF), smoking, diabetes mellitus, previous ischemic stroke and/or transient ischemic attack, peripheral vascular disease and myocardial infarction.
We defined arterial hypertension as systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg according to the criteria approved in 2013 by the European Society of Hypertension and by the European Society of Cardiology [32], or usage of antihypertensive drugs. We made a diagnosis of AF using standard electrocardiogram recording showing irregular RR intervals and no P waves lasting at least 30 seconds according to the criteria specified in the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology in 2010 [33] or AF documented earlier. We considered individuals as smokers if they smoked actively before index stroke or had smoked at least 100 cigarettes ever. We made diabetes mellitus diagnosis based on serum fasting glucose levels ≥ 7.0 mmol/L, serum glucose levels ≥ 11.1 mmol/L 2 h following the 75 g oral glucose tolerance test according to the World Health Organization criteria [34] or based on previously used blood glucose lowering drugs.
Ward nurses took blood samples for TAPH from all participating individuals on admission within 48 hours after AIS onset and again at the end of the first week of hospitalisation. We estimated subjects’ cognitive outcomes upon discharge from the neurology department (acute period) and repeatedly at the end of rehabilitation (subacute period) following AIS.
Assessment of mental state
We evaluated mental state using a validated Lithuanian version of the MMSE [35]. This questionnaire is an examiner-rated scale composed of eleven questions dedicated to assess orientation, memory, attention, ability to understand and execute commands, to write a sentence, and make a copy of complex figures, with the maximal score of 30 points [36]. We administered the MMSE at the end of the first hospitalization week and repeated it at the end of rehabilitation, usually 3–4 weeks later.
Evaluation of thyroid axis hormones
We measured serum levels of TSH, FT4 and FT3, taken on admission in a certified laboratory for all included individuals in the study. We also repeated measurements on eligible study participants upon discharge from neurology departments. Ward nurses carried out the second blood sampling in 142 (73.2%) individuals who completed the MMSE in the acute period and in 82 (42.3%) individuals who completed the MMSE in the subacute period. We excluded individuals from the second blood sampling most commonly because of their refusal to provide a blood sample.
A laboratory worker separated serum from the blood by centrifugation at 3,000 g and then placed it in the freezer at −70 °C. The analysis of TSH, FT4 and FT3 serum levels was performed simultaneously in all collected blood samples with electrochemiluminescence immunoassay analyser (Advia Centaur XP 2016; Siemens Osakeyhtiö, Espoo, Finland). We defined ranges of assessed hormones according to the laboratory specifications: the normal range for TSH was 0.55–4.78 mIU/L, for FT4 11.50–22.70 pmol/L and for FT3 3.50–6.50 pmol/L; subclinical hyperactive thyroid for TSH was < 0.550 mIU/L and FT4 as well as FT3 within normal levels; subclinical hypo-active thyroid for TSH was > 4.780 mIU/L and FT4 as well as FT3 within normal levels; low-T3 syndrome for FT3 < 3.50 pmol/L and TSH as well as FT4 within normal levels.
Statistical analysis
We performed statistical analyses using the SPSS (IBM, Armonk, NY, USA), version 25. We assessed data distribution using the Kolmogorov-Smirnov test and expressed the results as the mean ± standard deviation. Variables with non-normal distribution were expressed as the median with 25th–75th percentile (interquartile range). Repeatedly evaluated hormone levels were expressed as the difference between baseline TAPH levels and those measured on discharge. We calculated simple linear regression R2 and P values to identify included variables related to the MMSE total score during the acute and subacute AIS periods, respectively. We used variables with P < 0.2 later in multiple regression models. Homoscedasticity, multicollinearity and auto-correlation were eliminated using standard procedures. Furthermore, to evaluate the proportion of the variance in mental performance that accounted for the included variable, we applied a stepwise procedure. We reported estimates of unstandardized coefficients with standard error, standardized b and adjusted R2 in order to compare established correlates. All statistical tests were two-tailed. The statistical significance was selected at P < 0.05.
Results
Study participants characteristics
Figure 1 shows the flowchart of the study recruitment process. Before the thyroid profile evaluation, 347 individuals were dropped out during the recruitment time because they met the exclusion criteria of the study, leaving 265 individuals for further evaluation. Later, we ecluded 71 individuals from the study because of death, communication disorder or thyroid profile outside the accepted range. Finally, we included 194 individuals during an acute period and 89 individuals during the subacute AIS period for statistical analysis.
AIS – acute ischemic stroke; MMSE – Mini-Mental State Examination
Obr. 1. Schéma náboru účastníků studie.
AIS – akutní ischemická CMP; MMSE – Mini-Mental State Examination

Briefly, among 194 individuals with the first MMSE evaluation, the mean age was 67 (60–74) years, and 116 (59.8%) of them were men. Median baseline stroke severity according to the NIHSS score was 6 (4–11). Before index AIS, 182 (93.8%) of them had mRS estimate ≤ 2.
Among 89 individuals with the second MMSE evaluation, the mean age was 67 (59–74) years and 55 (61.8%) of them were men. The median NIHSS score was 7 (4–12) and 86 (96.6%) of them had a mild disability (mRS ≤ 2) before experienced AIS.
Individuals who remained in our study during the subacute period, were significantly less often treated with intravenous thrombolysis (P = 0.004), were less often diagnosed with AF (P = 0.046), had higher serum FT4 levels (P < 0.001) and had higher difference between baseline FT4 levels and those repeated on discharge (P < 0.001) compared to those (N = 105) who only completed it in the acute period.
Relationship between TAPH and mental state assessed by MMSE after experienced AIS
Univariate analysis of individuals who completed the first MMSE evaluation during the acute period showed that factors related to the MMSE estimate with P < 0.2 included age, stroke severity according to the NIHSS, disability before the experienced AIS evaluated with mRS, systolic arterial pressure, used anticoagulants or antiplatelets, AF, FT3 levels on admission, and difference between baseline FT4 levels and those repeated on discharge (Tab. 1). The same analysis of individuals who completed the second MMSE evaluation during the subacute period revealed that factors related to the MMSE estimate included age, NIHSS, AF, smoking, previous ischemic stroke and/or transient ischemic attack and FT3 levels (Tab. 2). These factors were later included into the multivariate analysis.
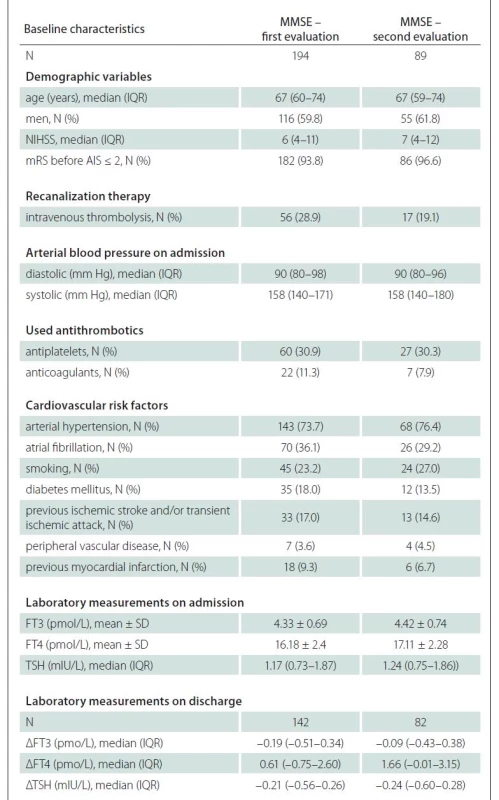
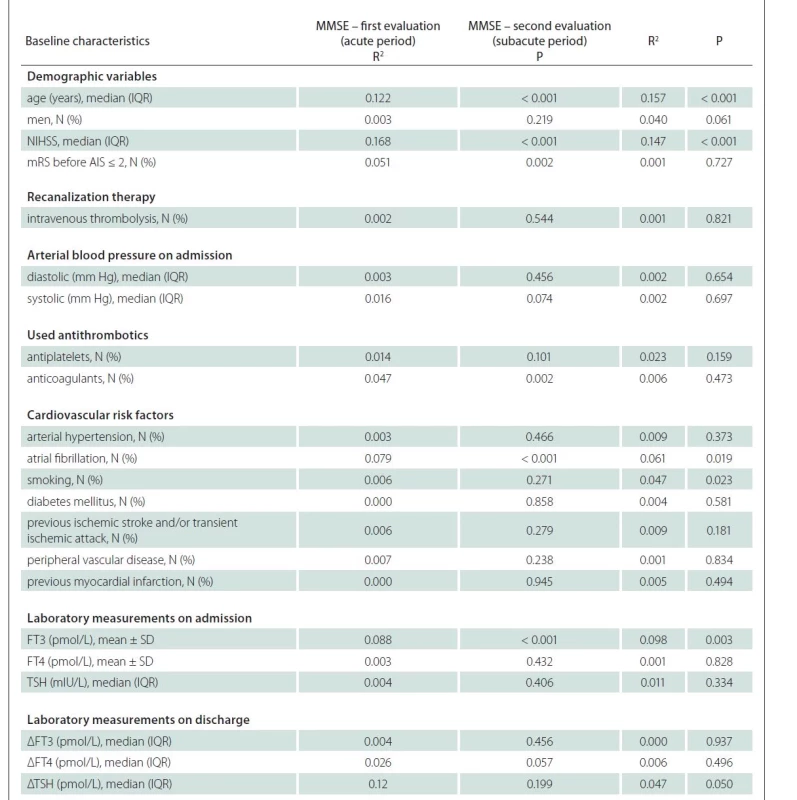
The stepwise linear regression performed in a sample of individuals who completed the first MMSE evaluation distinguished five factors associated with the MMSE: age, NIHSS, disability before AIS, FT3 levels on admission and use of anticoagulants (Tab. 3). Neurologic severity according to the NIHHS accounted for 16.3% of the variance in the MMSE, whilst other combined variables accounted for additional 18.9%. Here, FT3 change by 1 pmol/L accounted for 1.6% (R2 = 0.016; P = 0.017) of the total MMSE variability. The analysis showed that higher FT3 serum levels on admission were associated with higher MMSE estimate, while higher age, higher NIHSS, disability before AIS and use of anticoagulants led to opposite results.
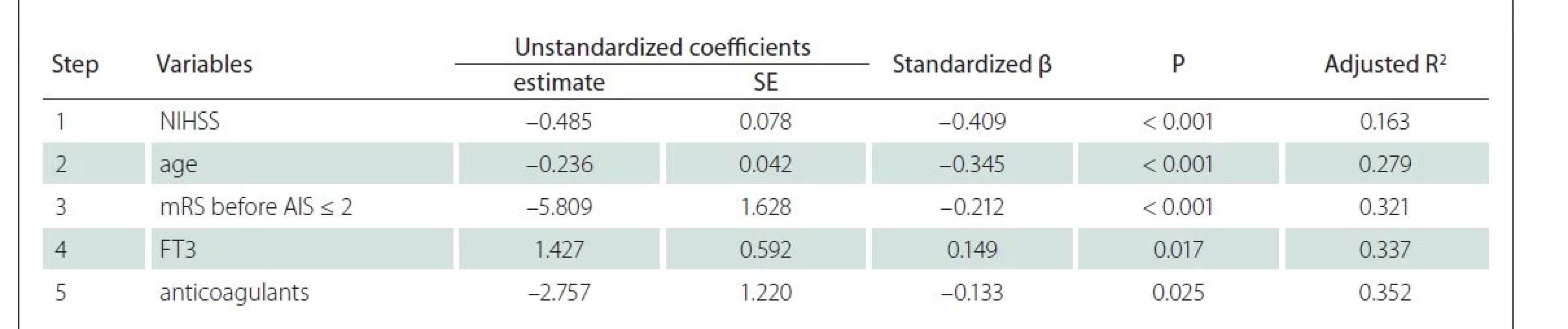
The stepwise linear regression of individuals who completed the second MMSE evaluation identified two factors associated with MMSE: age and NIHSS (Tab. 4). Both factors accounted for 28.2% of the variance in the MMSE.
Discussion
The intention of this work was to determine whether TAPH were associated with mental state after experienced AIS. We found significant associations between serum FT3 levels on admission and MMSE estimate during the acute AIS period: higher FT3 levels were related to a higher MMSE estimate. We did not find associations between TAPH and cognitive performance evaluated with MMSE during the subacute AIS period. We also did not identify associations between TAPH repeated derivatives and cognitive performance during both AIS periods.
There are few studies applied to the assessment of the association between TAPH and AIS cognitive outcomes. In 2014, Bunevicius et al published a study with fewer included individuals with AIS (N = 88) than in our study and their work established associations between lower FT3 levels and CIAS during discharge [28]. In 2018, Irimie et al presented a study that evaluated associations between serum TSH, total T3 and cognitive outcomes assessed by MMSE at discharge after experienced AIS [26]. This study did not identify independent associations between analysed TAPH and post-stroke cognitive outcomes. The results inconsistent with our results could be determined; firstly, measuring total T3 levels, not FT3 as in our study, which could be less associated with true hormonal state according to the free hormone hypothesis [37] and, secondly, including a lower number of subjects (N = 120) [26]. Another study, published by Chen et al in 2018, aimed to evaluate TSH, FT4 and FT3 associations with CIAS assessed by MMSE one month after experienced AIS, determined independent low-T3 syndrome associations with CIAS [27]. Our study did not show a relationship between FT3 and MMSE estimate during the subacute AIS period, possibly because many individuals dropped out of the study during this period.
The explanation of found associations between higher FT3 serum levels and better mental performance assessed by MMSE during the acute AIS period could be twofold. Firstly, during the last 50 years, there has been growing evidence that any severe (non-thyroidal) disease causes serum TAPH physiological-adaptation changes defined as non-thyroidal illness syndrome (NTIS) [38]. The mechanism of NTIS is complex and involves both the thyroid axis with reduced hypothalamic thyroliberin output and reduced thyroid follicle size as well as altered thyroid hormone metabolism in the peripheral tissue triggered by inflammatory cytokines and cortisol [39]. NTIS serum thyroid profile depends on the disease severity: the more severe the disease, the more pronounced TAPH changes. Decreased FT3 levels often indicate NTIS [38]. Due to this versatility encompassing different conditions, FT3 could just be a biomarker of the disease severity and thus related to its outcomes. The second explanation could be associated with the deleterious effect of lowered FT3 levels on a damaged brain, because the cerebral tissue is in constant need of optimal thyroid hormone levels. Post-ischemic-stroke recovery beginning within the first hours of its onset involves new synapse formation in the peri-infarct tissue, dendritic arborisation, and axonal sprouting including distal brain regions to form new neural connections [40]. There is also growing evidence for ischemic stroke incited neurogenesis for stroke recovery with the greatest potential of neurogenic niche located in the subventricular zone [41]. TH necessity for normal cognition is often reflected in clinical studies with established deleterious effect of adult onset of overt hypoactive thyroid on cognition, especially its memory domain [42]. Animal studies provide knowledge of TH deficiency having a harmful impact on brain morphology and functions with induced neuronal death and reduced synaptic plasticity in the hippocampal area [43]. With reference to TH effect on stroke recovery, there is growing evidence for their importance in various post-stroke recovery levels and steps [44]. A study of Talhada et al showed, that experimentally induced ischemic stroke in mice with intraperitoneally administered T3 during the acute period was associated with neuronal circuits reorganization, plasticity and accordingly with better outcomes [45]. Furthermore, this study provided new knowledge about defined TH receptor expression in the post-mortem human brain with differences observed in the infarct core showing changed thyroid signalling. Another experimental study with rodents showed a neuroprotective and antiedematous T3 effect in short term middle cerebral artery occlusion model of AIS with better functional outcomes [46]. As mentioned earlier, one of the stroke recovery pathways is associated with neurogenesis and here TH could play an important role because of established cell-autonomous role of TH action in adult rodent neurogenesis [47] and oligodendrogenesis [48]. Summarising these findings, we could propose possible detrimental effect of lowered serum FT3 levels on its availability in the cerebral tissue with a negative impact on stroke recovery including cognitive functions.
Strengths and limitations
Significant strengths of our work are related to the participation of three different centres and evaluation of TAPH at two different time points.
There are several shortcomings of the study. Firstly, our study did not include important factors for CIAS, such as stroke lesion characteristics, brain neurovisual changes consisting of brain atrophy and white matter disease, and pre-stroke cognitive status [49]. Secondly, we did not use neuropsychological instruments to exclude possible delirium, which is common in up to half of stroke cases during the acute period [50]. Thirdly, we evaluated cognitive functions using the MMSE questionnaire which has limited accuracy in determining cognitive impairments [51]. Fourthly, blood samples were collected at any time of the circadian cycle with the possibility of TAPH diurnal variation [52]. Fifthly, many individuals were lost during the follow-up. However, main characteristics, such as age, NIHSS and FT3 levels, did not differ significantly between those who had not participated in the follow-up and those who participated.
Conclusions
Our study established positive associations of FT3 serum levels on admission and MMSE estimate during the acute AIS period, but not during the subacute period. No associations were found between repeated TAPH values and MMSE estimates in both AIS periods.
This study further contributes to the confirmation that TH serum levels on admission could be associated with AIS cognitive outcomes. In our oppinion, the research focused on such associations should continue, at least for several reasons. First, such research could fill knowledge gaps linking TAPH other than mentioned in this study, including TH metabolites, and CIAS. In addition, studies with larger samples are needed for generalisability. Lastly, such research could contribute to the development of a panel of serum markers for AIS cognitive outcomes prognosis in daily practice.
Ethical aspects
Regional Biomedical Research Ethics Committee (permission numbers: P1-BE-2-11/2013 and P2-BE-2-11/2013) accredited the protocol of the study. We included study participants after written consent only. We took a written consent directly or from patient’s relatives in case of his/her inability to sign.
Funding
This study was funded by the European Social Fund under the Global Grant measure (grant number: VP1-3.1- SMM-07-K-02-060).
Conflicts of interest
The authors have no competing interests to report, except for J. B., who has served as a consultant to Cogstate, Ltd.
Saulius Taroza
Neuroscience Institute
Lithuanian University of Health
Sciences
Vydūno 4, Palanga, LT-00135
Lithuania
e-mail: saulius.taroza@lsmuni.lt
Accepted for review: 4. 2. 2022
Accepted for print: 1. 12. 2022
Sources
1. Barbay M, Diouf M, Roussel M et al. Systematic review and meta-analysis of prevalence in post-stroke neurocognitive disorders in hospital-based studies. Dement Geriatr Cogn Disord 2018; 46 (5–6): 322–334. doi: 10.1159/000492920.
2. Gallucci L, Umarova RM. Kognitive Defizite und Demenz nach Schlaganfall. Therapeutische Umschau 2021; 78 (6): 305–311. doi: 10.1024/0040-5930/a001278.
3. Mijajlović MD, Pavlović A, Brainin M et al. Post-stroke dementia – a comprehensive review. BMC Med 2017; 15 (1): 11. doi: 10.1186/s12916-017-0779-7.
4. Stolwyk RJ, Mihaljcic T, Wong DK et al. Poststroke cognitive impairment negatively impacts activity and participation outcomes: a systematic review and meta-analysis. Stroke 2021; 52 (2): 748–760. doi: 10.1161/strokeaha.120.032215.
5. Saposnik G, Cote R, Rochon PA et al. Care and outcomes in patients with ischemic stroke with and without preexisting dementia. Neurology 2011; 77 (18): 1664–1673. doi: 10.1212/WNL.0b013e31823648f1.
6. Das J, Rajanikant GK. Post stroke depression: the sequelae of cerebral stroke. Neurosci Biobehav Rev 2018; 90: 104–114. doi: 10.1016/j.neubiorev.2018.04.005.
7. Lee M, Saver JL, Hong KS et al. Cognitive impairment and risk of future stroke: a systematic review and meta-analysis. CMAJ 2014; 186 (14): E536–546. doi: 10.1503/cmaj. 140147.
8. Milinavičienė E, Rastenytė D, Kriščiūnas A. Effectiveness of the second-stage rehabilitation in stroke patients with cognitive impairment. Medicina 2011; 47 (9): 486–493.
9. Zietemann V, Georgakis MK, Dondaine T et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology 2018; 91 (20): e1838–e1850. doi: 10.1212/wnl.0000000000006 506.
10. Anstey KJ, Mack HA, von Sanden C. The relationship between cognition and mortality in patients with stroke, coronary heart disease, or cancer. Eur Psychol 2006; 11 (3): 182–195. doi: 10.1027/1016-9040.11.3.182.
11. Saa JP, Tse T, Baum C. Longitudinal evaluation of cognition after stroke – a systematic scoping review. PLoS One 2019; 14 (8): e0221735. doi: 10.1371/journal.pone. 0221735.
12. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8 (11): 1006–1018. doi: 10.1016/s1474-4422 (09) 70236-4.
13. Zhang X, Bi X. Post-stroke cognitive impairment: a review focusing on molecular biomarkers. J Mol Neurosci 2020; 70 (8): 1244–1254. doi: 10.1007/s12031-020-01 533-8.
14. Simpkins AN, Janowski M, Oz HS et al. Biomarker application for precision medicine in stroke. Transl Stroke Res 2020; 11 (4): 615–627. doi: 10.1007/s12975-019-00762-3.
15. Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke 2015; 46 (3): 915–920. doi: 10.1161/ strokeaha.114.005604.
16. Burkauskas J, Bunevicius A, Brozaitiene J et al. Cognitive functioning in coronary artery disease patients: associations with thyroid hormones, N-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein. Arch Clin Neuropsychol 2017; 32 (2): 245–251. doi: 10.1093/arclin/acx004.
17. Burkauskas J, Lang P, Bunevičius A et al. Cognitive function in patients with coronary artery disease: a literature review. J Int Med Res 2018; 46 (10): 4019–4031. doi: 10.1177/0300060517751452.
18. Balch MHH, Nimjee SM, Rink C et al. Beyond the brain: the systemic pathophysiological response to acute ischemic stroke. J Stroke 2020; 22 (2): 159–172. doi: 10.5853/jos.2019.02978.
19. Lamba N, Liu C, Zaidi H et al. A prognostic role for low tri-iodothyronine syndrome in acute stroke patients: a systematic review and meta-analysis. Clin Neurol Neurosurg 2018; 169: 55–63. doi: 10.1016/j.clineuro.2018.03.025.
20. Wang F, Luo MY, Zhou L et al. Endocrine dysfunction following stroke. J Neuroimmune Pharmacol 2021; 16 (2): 425–436. doi: 10.1007/s11481-020-09935-6.
21. Taroza S, Rastenyte D, Burkauskas J et al. Lower serum free triiodothyronine levels are associated with symptoms of depression after ischemic stroke. J Psychosom Res 2019; 122: 29–35. doi: 10.1016/j.jpsychores. 2019.04.018.
22. Taroza S, Rastenytė D, Podlipskytė A et al. Nonthyroidal illness syndrome in ischaemic stroke patients is associated with increased mortality. Exp Clin Endocrinol Diabetes 2020; 128 (12): 811–818. doi: 10.1055/ a-0915-2015.
23. Giannocco G, Kizys MML, Maciel RM et al. Thyroid hormone, gene expression, and central nervous system: where we are. Semin Cell Dev Biol 2021; 114: 47–56. doi: 10.1016/j.semcdb.2020.09.007.
24. Papaefthymiou O, N‘Guyen S, Smith C et al. Dysfonction thyroïdienne et fonctions cognitives: mythe ou réalité? Praxis (Bern 1994) 2016; 105 (20): 1205–1212. doi: 10.1024/1661-8157/a002485.
26. Irimie CA, Vârciu M, Irimie M et al. C-reactive protein and T3: new prognostic factors in acute ischemic stroke. J Stroke Cerebrovasc Dis 2018; 27 (10): 2731–2737. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.047.
27. Chen H, Wu Y, Huang G et al. Low tri-iodothyronine syndrome is associated with cognitive impairment in patients with acute ischemic stroke: a prospective cohort study. Am J Geriatr Psychiatry 2018; 26 (12): 1222–1230. doi: 10.1016/j.jagp.2018.07.007.
28. Bunevicius A, Kazlauskas H, Raskauskiene N et al. Ischemic stroke functional outcomes are independently associated with C-reactive protein concentrations and cognitive outcomes with triiodothyronine concentrations: a pilot study. Endocrine 2014; 45 (2): 213–220. doi: 10.1007/s12020-013-9958-2.
29. Burkauskas J, Brozaitiene J, Staniute M et al. Gene-environment interactions connecting low triiodothyronine syndrome and outcomes of cardiovascular disease (GET-VASC): study protocol. Biol Psychiatr Psychopharmacol 2014; 16 (2): 66–73.
30. Kazukauskiene N, Skiriute D, Gustiene O et al. Importance of thyroid hormone level and genetic variations in deiodinases for patients after acute myocardial infarction: a longitudinal observational study. Sci Rep 2020; 10 (1): 9169. doi: 10.1038/s41598-020-66006-9.
31. Aho K, Harmsen P, Hatano S et al. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ 1980; 58 (1): 113–130.
32. Mancia G, Fagard R, Narkiewicz K et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2014; 23 (1): 3–16. doi: 10.3109/08037051.2014.868629.
33. Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31 (19): 2369–2429. doi: 10.1093/eurheartj/ehq278.
34. World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. [online]. Dostupné z: https: //apps.who.int/iris/handle/10665/43588.
35. Bunevicius R. Protinės būklės mini tyrimas. Biol Psychiatr Psychopharmacol 2000; 2 (1): 13.
36. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12 (3): 189–198. doi: 10.1016/0022-3956 (75) 90026-6.
37. Bikle DD. The free hormone hypothesis: when, why, and how to measure the free hormone levels to assess vitamin D, thyroid, sex hormone, and cortisol status. JBMR Plus 2021; 5 (1): e10418. doi: 10.1002/jbm4.10418.
38. Fliers E, Boelen A. An update on non-thyroidal illness syndrome. J Endocrinol Invest 2021; 44 (8): 1597–1607. doi: 10.1007/s40618-020-01482-4.
39. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin 2019; 35 (2): 375–388. doi: 10.1016/ j.ccc.2018.11.012.
40. Grefkes C, Fink GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract 2020; 2: 17. doi: 10.1186/s42466-020-00060-6.
41. Xie F, Liu H, Liu Y. Adult neurogenesis following ischemic stroke and implications for cell-based therapeutic approaches. World Neurosurg 2020; 138: 474–480. doi: 10.1016/j.wneu.2020.02.010.
42. Samuels MH. Thyroid disease and cognition. Endocrinol Metab Clin North Am 2014; 43 (2): 529–543. doi: 10.1016/j.ecl.2014.02.006.
43. Fernández-Lamo I, Montero-Pedrazuela A, Delgado--García JM et al. Effects of thyroid hormone replacement on associative learning and hippocampal synaptic plasticity in adult hypothyroid rats. Eur J Neurosci 2009; 30 (4): 679–692. doi: 10.1111/j.1460-9568.2009.06862.x.
44. Talhada D, Santos CRA, Goncalves I et al. Thyroid hormones in the brain and their impact in recovery mechanisms after stroke. Front Neurol 2019; 10: 1103. doi: 10.3389/fneur.2019.01103.
45. Talhada D, Feiteiro J, Costa AR et al. Triiodothyronine modulates neuronal plasticity mechanisms to enhance functional outcome after stroke. Acta Neuropathol Commun 2019; 7 (1): 216. doi: 10.1186/s40478-019-0866-4.
46. Sadana P, Coughlin L, Burke J et al. Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: possible association with AQP4 modulation. J Neurol Sci 2015; 354 (1–2): 37–45. doi: 10.1016/ j.jns.2015.04.042.
47. Mayerl S, Heuer H, Ffrench-Constant C. Hippocampal neurogenesis requires cell-autonomous thyroid hormone signaling. Stem Cell Reports 2020; 14 (5): 845–860. doi: 10.1016/j.stemcr.2020.03.014.
48. Remaud S, Demeneix B. Thyroid hormones regulate neural stem cell fate. Biol Aujourdhui 2019; 213 (1–2): 7–16. doi: 10.1051/jbio/2019007.
49. Mok VC, Lam BY, Wong A et al. Early-onset and delayed-onset poststroke dementia – revisiting the mechanisms. Nat Rev Neurol 2017; 13 (3): 148–159. doi: 10.1038/nrneurol.2017.16.
50. Shi Q, Presutti R, Selchen D et al. Delirium in acute stroke: a systematic review and meta-analysis. Stroke 2012; 43 (3): 645–649. doi: 10.1161/strokeaha.111.643726.
51. Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009; 43 (4): 411–431. doi: 10.1016/j.jpsychires.2008.04.014.
52. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol 2012; 349 (1): 91–104. doi: 10.1016/j.mce.2011.09.003.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2023 Issue 1
Most read in this issue
- Progressive multiple sclerosis in the light of the latest findings
- Dietary approaches specific to patients with multiple sclerosis
- Recommendations for structural brain MRI in the diagnosis of epilepsy
- Stroke specific measurement tools used to assess health related quality of life in young adults after ischemic stroke
