Effect of sumatriptan following simulated traumatic brain injury in rats
Účinek sumatriptanu po simulovaném traumatickém poranění mozku u potkanů
Úvod: Traumatické poranění mozku (traumatic brain injury; TBI) je v rozvojových zemích stále velmi vážným problémem. Nevyhnutelným výsledkem traumatu je sekundární fáze s neuroinflamací, která vede ke zvýšené morbiditě a mortalitě. Do současné doby byly tetovány různé účinné látky a léčebné modality. První klinicky dostupný tryptan, který byl použit k léčbě migrény – sumatriptan (SUM) – byl předtím hodnocen při cerebrální, testikulární a renální ischemii, ale pokud je nám známo, toto je první článek, který hodnotí jeho protizánětlivé a antioxidační vlastnosti po experimentálním TBI u potkanů. Cíl: Při použití naší techniky experimentálního traumatu jsme si dali za cíl zjistit, zda SUM zlepšuje stav při zánětlivé fázi TBI, a to prostřednictvím neurologické, histologické a biochemické analýzy. Metodologie: Dvacet tři potkanů kmene Wistar albino bylo náhodně rozděleno do tří skupin: kontrolní skupina, skupina s traumatem a skupina s traumatem, které byl podáván SUM. U posledních dvou skupin bylo vyvoláno experimentální difuzní poranění kortexu, které simulovalo TBI. Subjekty podstoupily neurologické hodnocení prostřednictvím Garciova testu, histologické vyšetření pomocí nového skórovacího systému a biochemickou analýzu hladin neuron specifické enolázy (NSE), S100B, kaspázy-3 (CASP3) a tzv. „rat thiobarbituric acid reactive substances“ (TBARS). Výsledky: Skupina, které byl podáván SUM, měla lepší skóre Garciova testu (p < 0,001), zvýšený protizánětlivý účinek (p = 0,004) a neuroprotektivní účinek (p < 0,001) a zároveň sníženou hladinu TBARS (p < 0,05), ale v porovnání se skupinou s traumatem měly podobné hladiny S100B, CASP3 a NSE. Závěr: Podání SUM může přinést prospěch z hlediska snížení mortality a morbidity po TBI vlivem jeho protizánětlivého a antioxidačního účinku. Nový skórovací systém použitý v této studii může být u podobných experimentů užitečným nástrojem.
Klíčová slova:
trauma – poranění mozku – sumatriptan – neuroprotektivní – protizánětlivý – antioxidační
Authors:
I. Bozkurt 1; S. Senturk 2; M. E. Yaman 3; Y. Guvenc 4; Y. Ozturk 5; G. Guney 6; M. Cingirt 7; O. Gulbahar 8
Authors‘ workplace:
Neurosurgery Clinic, Cankiri State, Hospital, Cankiri, Turkey
1; Neurosurgery Clinic, Istanbul Bahcelievler, Memorial Hospital, Istanbul, Turkey
2; Department of Neurosurgery, Gazi, University Hospital, Ankara, Turkey
3; Department of Neurosurgery, Marmara, University Hospital, Istanbul, Turkey
4; Department of Neurosurgery, Yenimahalle, Training and Research Hospital, Ankara, Turkey
5; Department of Pathology, Hitit University, School of Medicine, Çorum, Turkey
6; Department of Medical Biochemistry, Rize State Hospital, Rize, Turkey
7; Department of Medical Biochemistry, Faculty of Medicine, Gazi University, Ankara, Turkey
8
Published in:
Cesk Slov Neurol N 2022; 85(5): 389-396
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn2022389
Overview
Introduction: Traumatic brain injury (TBI) continues to be a devastating problem in developing countries. The inevitable traumatic effect is followed by a secondary phase of neuroinflammation leading to increased morbidity and mortality. Numerous agents and treatment modalities have been tested to date. The first clinically available triptan used to treat migraine, sumatriptan (SUM), has been evaluated for cerebral, testicular and renal ischemia previously but to our knowledge this is the first paper where its anti-inflammatory and anti-oxidative effects after experimental TBI in rats has been evaluated. Aim: Employing an experimental trauma technique, we aimed to investigate whether SUM has ameliorating effects on the inflammatory phase of TBI via neurological, histological and biochemical analyses. Methodology: Twenty-three Wistar albino rats were randomly divided into three groups: control, trauma and trauma + SUM. The two latter groups underwent experimental diffuse cortical injury mimicking TBI. The subjects underwent neurological assessment via the Garcia Test, histological analysis via a novel scoring system and biochemical analyses of neuron specific enolase (NSE), S-100B, caspase-3 (CASP3), and rat thiobarbituric acid reactive substances (TBARS) levels. Results: SUM receiving group had a better Garcia Test score (P < 0.001), higher anti-inflammatory score (P = 0.004) and higher neuroprotective score (P < 0.001) along with a better histopathological score when compared with the trauma group. SUM receiving group had lower levels of S100B, CASP3 and TBARS. SUM was unable to reduce NSE levels. Conclusion: SUM may prove to be beneficial in decreasing mortality and morbidity rates after TBI through its anti-inflammatory and anti-oxidative effects. The novel scoring system used in this study may be a valuable tool for similar experiments.
Keywords:
trauma – brain injury – sumatriptan – neuroprotective – anti-infl ammatory – anti-oxidative
Introduction
According to the United States Centers for Disease Control and Prevention (CDC), traumatic brain injury (TBI) is defined as a blow or jolt to the head or a penetrating head injury that disrupts the normal function of the brain. Any form of external force applied directly or indirectly to the head can cause TBI via stretching, tearing or compression of the brain tissue, which results in the loss of neural tissue. Following this inevitable primary phase, damage-associated molecular patterns (DAMPs) are released, thus inducing a cascade of metabolic, biochemical and inflammatory responses with the activation of microglia and peripheral leukocytes. This immune response in turn causes cell death via neuroinflammation and brain edema making up the secondary phase of TBI [1]. The secondary phase of TBI may last for months, thereby contributing to long-term deteriorating effects. Although the primary phase is inevitable, ameliorating neurological deficits caused by neuroinflammation during the secondary phase of TBI via suppressing detrimental immune responses has proven to be effective [2,3].
Approximately 3 million emergency room visits, deaths and hospitalization due to TBI occur in the United States alone. Similar but varying numbers are present in Europe whereas developing countries have a higher incidence of TBI-related mortality and morbidity at 160 per 100,000 persons in India and 344 per 100,000 in Asia. It is predicted that by the year 2030, TBI-related injury and disability will exceed many diseases in the developing world. With trauma centers reporting as high as 50% mortality rates due to TBI amongst all trauma-related deaths [4], it continues to pose a public health care concern. Developing countries have a higher incidence of motor vehicle-related TBI, whereas developed countries have a higher incidence of TBI due to falls especially in the elderly group [4]. Regardless of the cause, TBI has long-term, generally irreversible devastating effects on normal brain functions causing a burden for the patient, the family and the health care system.
Although many agents including coffee have been tested in preclinical studies with promising results, none have made it into clinical use [5,6]. Thus, there is a desperate need for an agent to attenuate injury caused by the secondary phase of TBI. Sumatriptan (SUM), the first clinically available triptan used to treat migraine, a selective agonist of serotonin 5-hydroxytryptamine 1B, has 1D (5HT1B, 1D) receptors that decrease the release of neurotransmitters and neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P (SP) by acting on the serotonin receptors at the terminal ends of neurons [7]. This in turn exerts an anti-inflammatory and anti-oxidative response. Other studies have shown SUM’s anti-inflammatory effect by the inhibition of neurogenic inflammation of the dural vessels and its anti-oxidative effect via increasing superoxide dismutase (SOD) [8].
Previous studies evaluating SUM’s effect on cerebral [9], myocardial [10] and testicular [8] ischemia, peripheral neuropathy [7], skin-flap survival [11], and post-traumatic headache (PTH) [12,13] have yielded promising favorable results. Also, studies focusing on serotonin’s effect on TBI and its sequel have produced positive effects [14,15]. A systematic review by Yue et al [14] found that selective serotonin reuptake inhibitors (SSRI) after TBI were beneficial in decreasing neuropsychiatric disorders. Li et al [15] concluded that N-acetyl serotonin improved neurogenesis and ameliorated cognitive impairment after experimental TBI. Despite these above-mentioned studies, the effects of SUM after experimental TBI have not been investigated yet. To our knowledge, no previous study has evaluated SUM’s anti-inflammatory response after TBI, hence this study aimed to be the first paper to discuss its effects via neurological, biochemical and histological analyses.
Materials and methods
Animals
Twenty-three healthy male Wistar albino rats ranging from 250–320 g were used in the study. They were maintained in a temperature‑controlled room (24 ± 2 °C) on a 12‑h light, 12‑h dark cyclical sequence and were given free access to standard bait and water. The subjects were randomly divided into three groups: control (N = 8), trauma (N = 7) and SUM (N = 8).
Procedure & drug administration
In order to induce TBI in the subjects, the method described by Marmarou et al [16] for diffuse cortical injury with minor modifications was used. The subjects were given 60 mg/kg of ketamine hydrochloride IP (Alfamine 10%, Egevet Veterinary Services, Famagusta, Cyprus) and 5 mg/kg of xylazine (Alfazyne 2%, Egevet Veterinary Services) for general anesthesia. The subjects were placed in a prone position on the table; a midline scalp incision was made to expose the coronal and lambdoid sutures. A metal disc with a 10- mm diameter and 3- mm thickness was fixated onto the cranium using bone wax. A cylindrical tube measuring 70 cm in height was used to drop a similar shaped lead object weighing 450 g onto the cranium through the tube. Subjects in the control group only received anesthesia, the trauma group subjects were administered 2 mL of 0.9% NaCl immediately after the trauma and again on the following day. The subjects in the SUM group were administered SUM at a dosage of 30 mg/kg via intranasal puff immediately after the trauma and on the following day.
Neurological assessment
All of the subjects were observed for a week and evaluated with the Garcia Test [17]. The Garcia Test is a composite neurological test in which the rats are evaluated for various sensorimotor deficits. The six tests used were spontaneous activity, symmetry in movement of the four limbs, forepaw outstretching, climbing, body proprioception and response to vibrissae touch. Each factor has a maximum score of 3, whereby the maximum Garcia Test score is 18.
Histological analysis
Subsequently, the subjects were given anesthesia with the above-mentioned agents and the brains were extracted immediately without any damage. Samples of neural tissue were obtained from three different planes covering the frontoparietal lobes bilaterally (Fig. 1) and were subjected to histopathological and biochemical analyses.
Obr. 1 Oblasti, ze kterých byly odebrány vzorky k hodnocení morfologických změn.
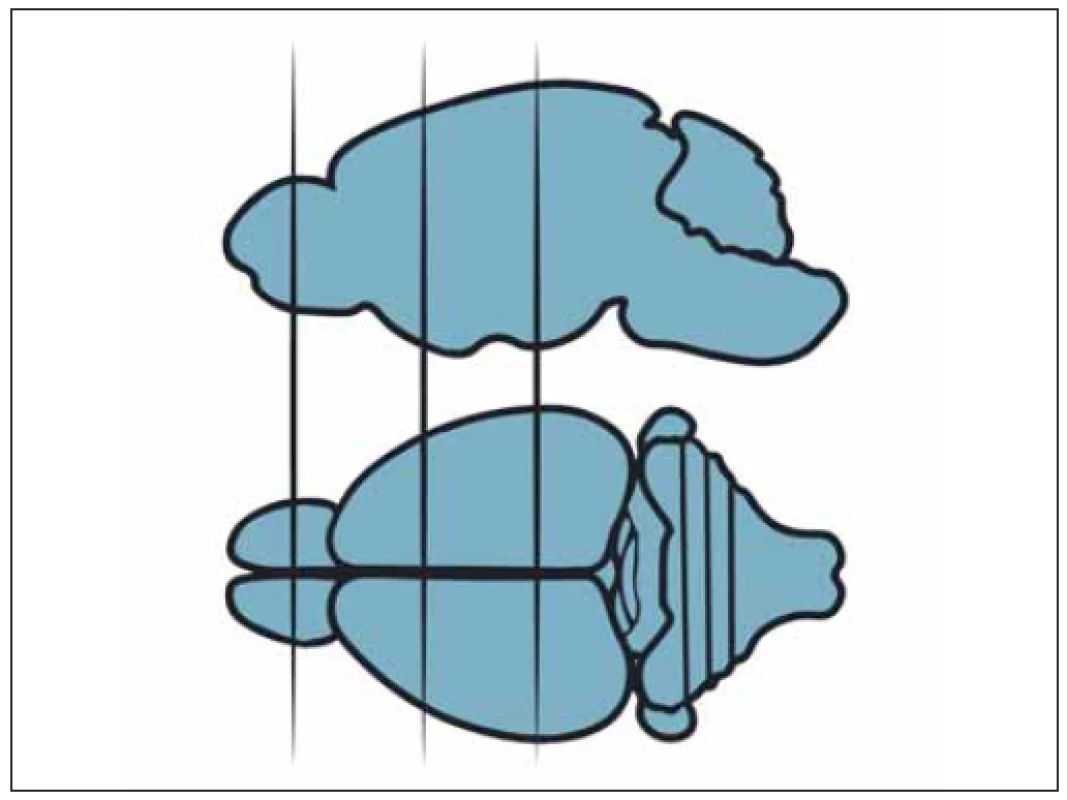
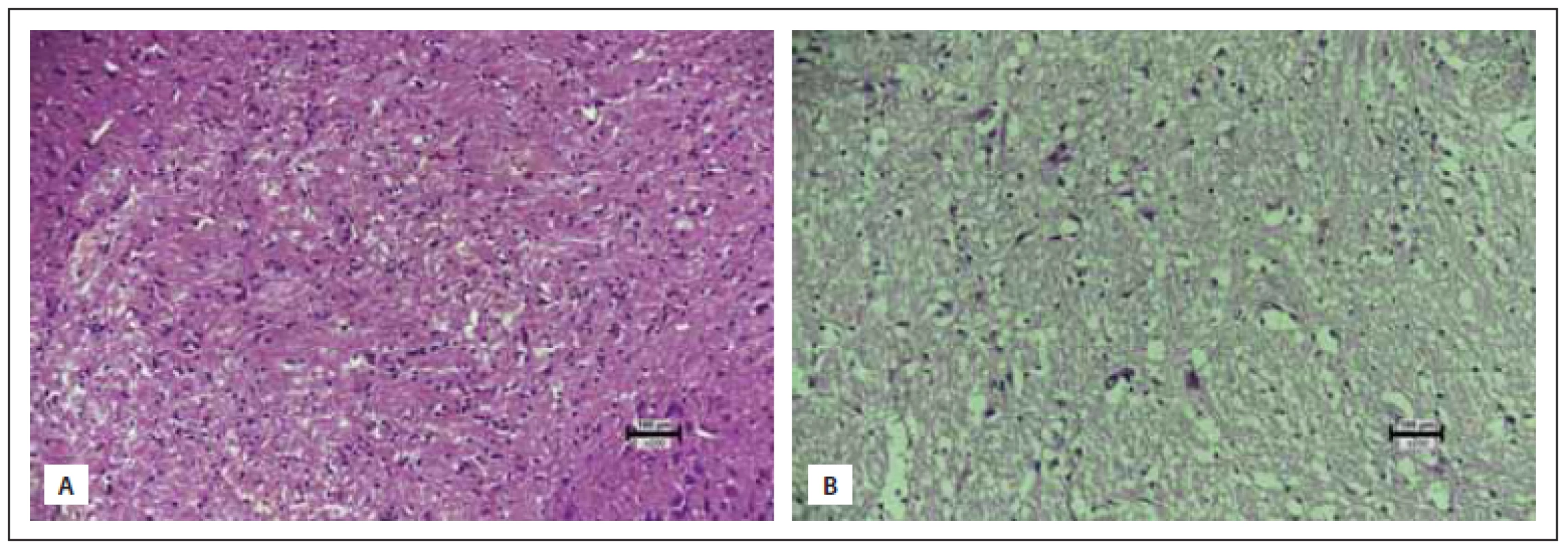
All obtained samples were kept in 10% buffered formaldehyde for 24 h. After the fixation process, macroscopic sampling was performed. The tissues were cased in 4- mm thick slices in the sagittal axis. Then, tissue fixation was achieved through immersion in an alcohol bath for 24 h. Tissues embedded in paraffin cassettes were cut into 5-micron slices with a microtome, stained with hematoxylin eosin (HE) and evaluated under a light microscope (Nikon Eclipse Ci, Minato, Japan). The samples were analyzed for anti-inflammatory and neuroprotective effect of the agent used by evaluating neuronal loss, gliosis, inflammation and congestion, in which each variable was assigned from 0 to 3 points. Congestion and neuronal loss were evaluated in 10 different magnification areas (400×) and the average of the total score was taken for each specimen. For inflammation and gliosis, all sections of the specimens were evaluated. Inflammation and congestion constituted the anti-inflammatory effect whereas neuronal loss and gliosis determined the neuroprotective effect summing up the total histopathological score. The maximum score was determined to be 12, constituting healthy tissue and the minimum score was zero (Tab. 1, Fig. 2–5). All of the upper and lower limits along with ranging values were identified via control (healthy: 9 pts.) and most damaged tissues (0 pts.). To the best of our knowledge, this is the first organized histopathological scoring system used in experimental head trauma.
Obr. 2 (A) Významná ztráta neuronů a degenerace neuronů ve skupině s traumatem (skóre 0). (B) Ve skupině na sumatriptanu jsou sice
neurony zachovány, ale pokračuje degenerace (skóre 3) (hematoxylin-eozin 200×).
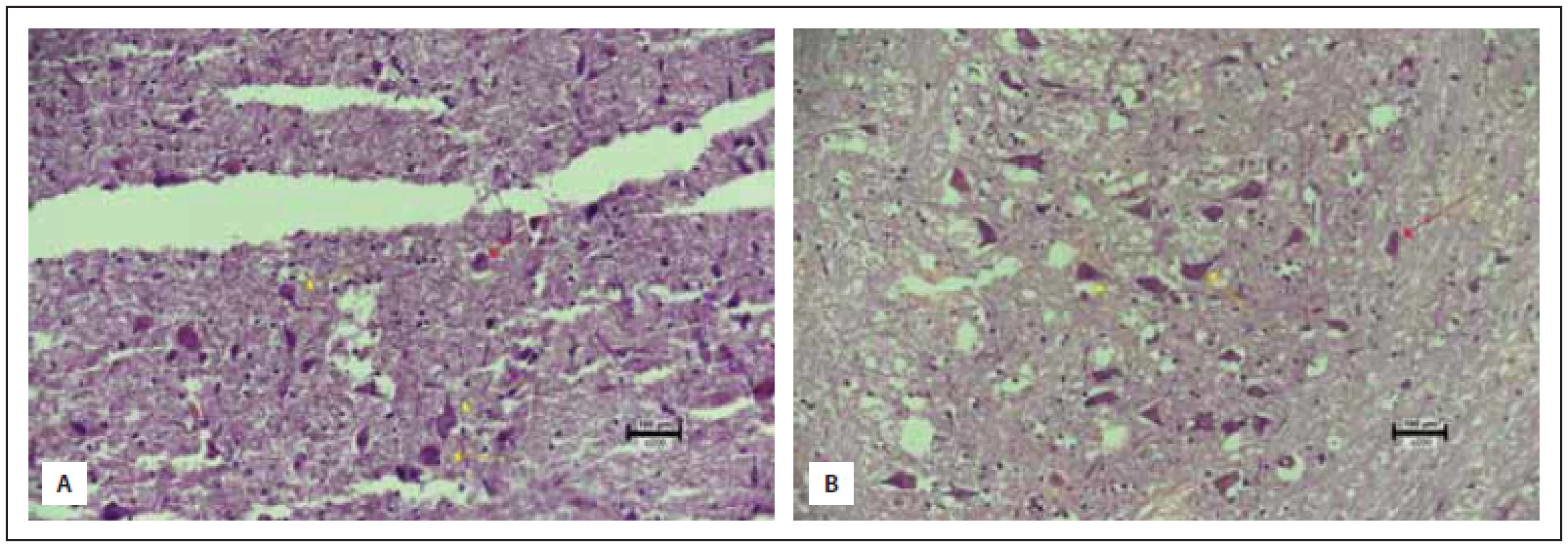
žluté šipky – degenerované neurony s hypertrofi í jader a degenerací chromatinu; červené šipky – pravidelné obrysy jader a normální distribuce
chromatinu
Obr. 3 (A) Skupinky lymfoidních buněk v mozkové tkáni ve skupině s traumatem (skóre 0). (B) Několik dispergovaných lymfoidních buněk
v mozkové tkáni ve skupině na sumatriptanu (skóre 1) (hematoxylin-eozin 200×).
Obr. 4 (A) Dvě utlačené vaskulární struktury na 1 hpf ve skupině s traumatem (skóre 1). (B) Jedna utlačená struktura na 1 hpf ve skupině
na sumatriptanu (skóre 2) (hematoxylin-eozin 200×).
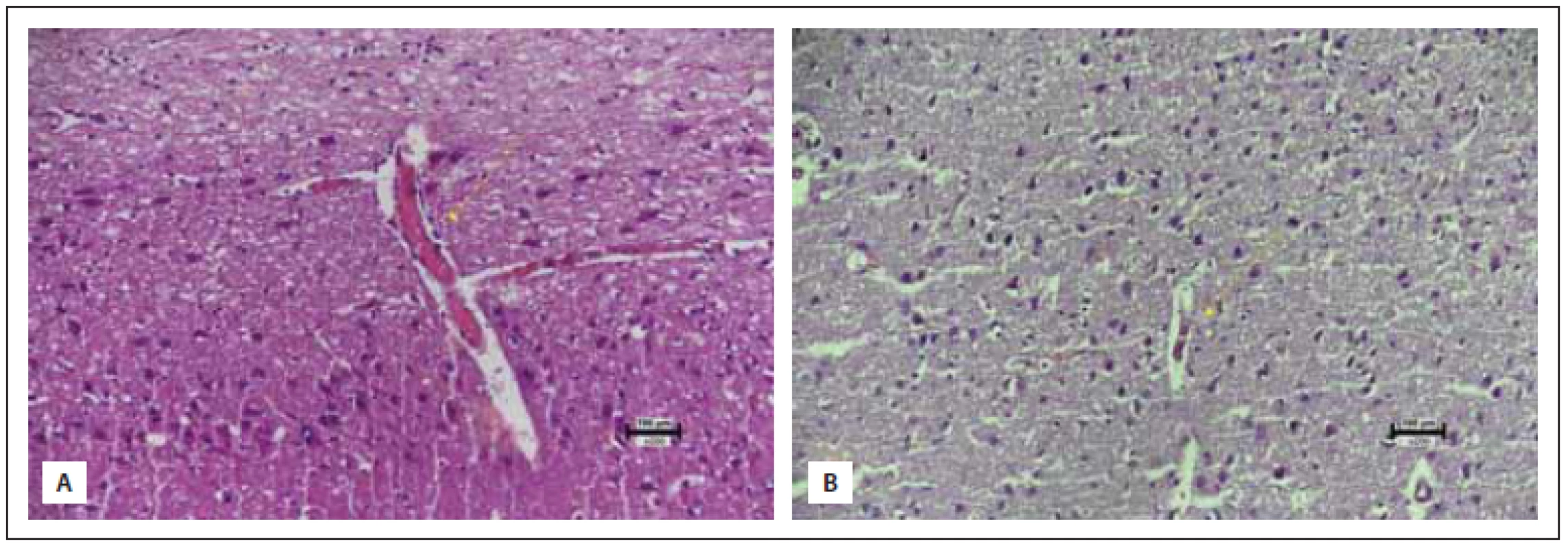
(A) Normální mozková tkáň v kontrolní skupině (skóre 3).
(B) Významná glióza ve skupině s traumatem (skóre 0). (C) Mírná
glióza ve skupině na sumatriptanu (skóre 2) (hematoxylin-eozin
200×).
While evaluating neuronal loss, the number of neurons in a high-power field (hpf) of 5 were counted in the control group and were averaged (65 neurons). The trauma and treatment group were compared to this. While evaluating gliosis, the control group constituted a score of 3 (normal), and the case with the highest amount gliosis score was determined to be zero. All other cases were evaluated in between these reference points.
Biochemical analysis
The tissue samples were subjected to biochemical analysis by a blinded biochemistry consultant in order to evaluate neuron specific enolase (NSE), S-100B, caspase-3 (CASP3), and rat thiobarbituric acid reactive substances (TBARS). The tissue samples were centrifuged after a homogenous aqueous mixture was obtained with a solution of 0.9% sodium chloride. The supernatant of these mixtures was subjected to a commercial solid-phase enzyme immunoassay kit (SHANGHAI YEHUA Biological Technology Co., Ltd, Shanghai, China). These markers are known to be increased in serum and CSF levels after trauma [18–21].
Statistical analysis
All statistical analyses were performed using SPSS, version 17.0 (IBM, Armonk, NY, USA). The results were tested for normality and where there was no normal distribution, the Mann-Whitney U test was used and the minimum, maximum and median values were derived. Where normal distribution was present, Student’s T test was used for statistical evaluation of the data. Statistical significance was accepted as P < 0.05.
Results
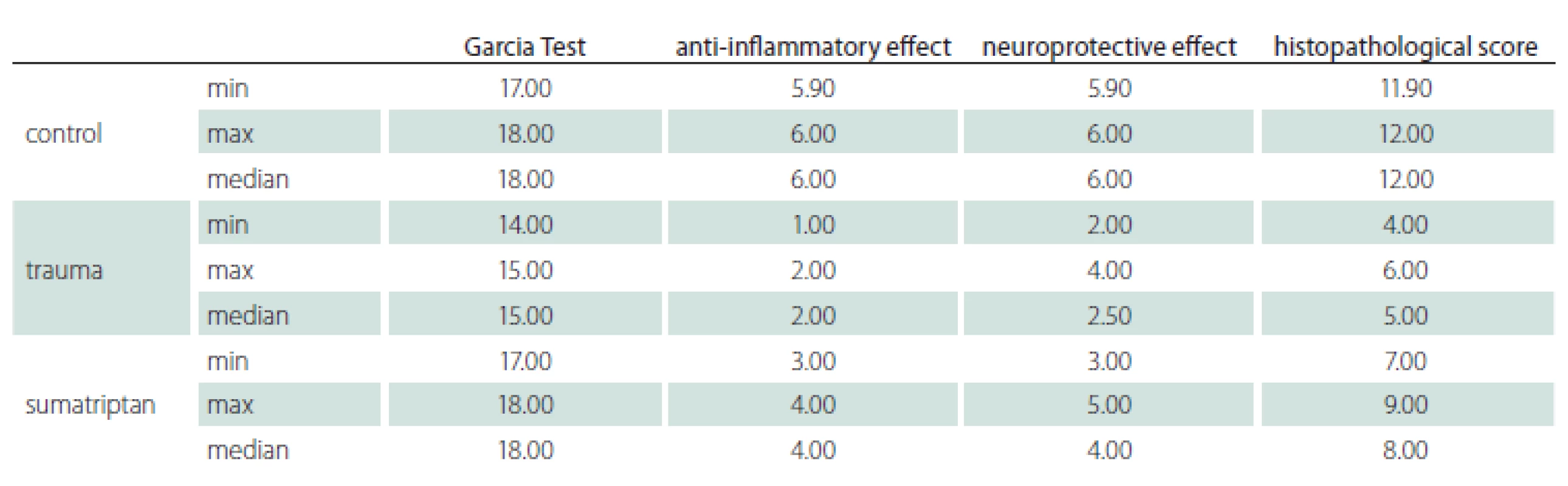
The minimum, maximum and median values of the Garcia Test, anti-inflammatory and neuroprotective effect along with total histopathological score are listed in Tab. 2. The trauma group had a worse Garcia Test score along with histopathological parameters and it was statistically significant (P < 0.001). The SUM group had a better Garcia Test score along with histopathological parameters and it was statistically significant (P < 0.001).
The mean values of the biochemical analyses of the groups were compared and are summarized in Tab. 3. The trauma group had statistically significantly higher NSE, S100B, CASP3 and TBARS levels when compared with the control group. SUM did not show a beneficial effect on NSE when compared to the trauma group. It decreased S100B, CASP3 and TBARS levels compared to the trauma group although only the decrease in TBARS was statistically significant. The agent was able to reduce the parameters close to the control group, but only CASP3 and TBARS comparison revealed statistically significant results. This is most likely due to a low sample size and a high standard deviation.

Discussion
The principle aim of this study was to evaluate SUM’s effect after TBI via its anti-inflammatory effects. SUM exerts its anti-inflammatory effect by decreasing the release of SP and CGRP by acting on the terminal ends of neurons through 5HT1B/1D receptors [22]. Inflammatory response evoked by trauma plays a crucial role in the progression of TBI. As evidenced by this paper and supported by the results of previous studies, NSE, S-100B, CASP3 and TBARS levels increase after trauma amongst other markers [18–21]. This correlates with the inflammatory response and in turn corresponds to the histopathological findings such as neuronal loss, gliosis and congestion. Clinical entities of these findings were evidenced in this paper via neurological assessment of subjects using the Garcia Test.
Previous studies evaluating SUM’s effect used ranging values of 0.1–5 mg/kg [7,9,23]. This study used a higher rate of 30 mg/kg similar to the dose used for migraine attacks with an interval of 24 h as advised. A study evaluating the efficacy of SUM has proved higher doses to be more effective in treating migraine attacks [24]; in contrast to previous studies, a higher dose was employed.
In the treatment of migraine, SUM’s effect of vasoconstriction plays a crucial role in decreasing the cerebral blood flow along with the constriction of dural and meningeal vessels [25]. This factor alone may play a crucial role in preventing the vasodilatation causing the inflammatory process following trauma, decreasing edema and the migration of pro-inflammatory cytokines. Another pathway in which SUM alleviates migraine is the activation of 5‑HT1B/1D receptors leading to decreased cyclic adenosine monophosphate (cAMP) resulting in the inhibition of SP, CGRP, glutamate and other inflammatory mediators’ release [8]. Migraine itself is a pro-inflammatory response, thus using an agent which targets decreasing this inflammatory response may also prove to be beneficial in regulating the inflammatory response after TBI.
The injury model used in this study proposed by Marmarou et al [16] aimed to simulate the most common form of TBI: diffuse axonal head injury (DAI). DAI in humans is mostly caused by falls and motor vehicle accidents, making it suitable to evaluate a target therapy’s effect. This technique allows for a simple, reproducible and practical model of a closed head injury without brain-stem damage or focal brain lesions. The reason why this technique has been used is that it has been shown to mimic closed head trauma in humans with posttraumatic ventriculomegaly in survivors, and more than 1,500 papers have cited this technique. In an extensive and comprehensive review of animal models of TBI, Marmarou’s technique was described to be one of the closest models in mimicking human TBI by causing widespread damage of neurons, axons, dendrites and microvasculature along with DAI. It was also able to cause the motor and cognitive deficits with difficulties in beam walking and memory [26].
NSE, an enzyme released from neurons after the injury, plays a pivotal role in cerebral glycolytic energy metabolism which makes it a suitable marker of TBI [27]. NSE concentrations are high in neurons and neuroendocrine cells. This marker has also been evaluated in stroke and subarachnoid hemorrhage yielding elevated results [18]. The cellular degradation caused by the trauma and thereafter due to apoptosis along with cerebral edema is the main cause of NSE elevation after TBI. However, it remains unknown whether NSE increase after TBI is solely due to the neuronal damage or a response to the inflammatory process. Although the findings of this study were parallel to previous findings where increased NSE levels were detected in the trauma group, SUM differed in not being able to decrease NSE levels. The authors of this paper have postulated that SUM through its anti-inflammatory effects may be able to regulate the inflammatory response of TBI. However, NSE is elevated after the proliferation of neurons in malignancies and cellular damage after infarction or trauma [28]. The production of NSE is not initiated by an inflammatory response; it rather proves to be beneficial in evaluating the extent of trauma which would explain why SUM was unable to reduce NSE levels. Studies analyzing a longer period of recovery after TBI may find reduced levels of NSE, which may correlate with a decreased inflammatory response, hence a decreased rate of cellular damage.
A small Ca+2 binding protein, S100B, is expressed in astrocytes, Schwann cells and oligodendrocytes. It promotes neuroprofileration, differentiation and cytoskeleton assembly, all of which are involved in neural regeneration after trauma via inflammation. However, its extracellular concentrations determine a neurotrophic or a neurotoxic effect [29]. S100B was found to be higher in trauma patients and in numerous neurological conditions, thus making it a valuable marker after TBI. Increased levels were correlated with the injury severity and were predictive for unfavorable outcome after TBI along with elevated CSF concentrations in patients with brain death due to TBI [30]. Both NSE and S100B correlated with better Glasgow Coma Scale (GCS) scores in pediatric patients after TBI [31]. Although S100B is involved in the proliferation, differentiation, Ca+2 homeostasis and energy metabolism, it has been shown to be closely associated with the inflammatory response, oxidative stress and apoptosis [32]. This study revealed similar results where S100B decrease was found in the treatment group compared to the trauma group and similar to the control group, but was not statistically significant. Nonetheless, SUM presumably decreased the inflammatory response after TBI allowing the down regulation of S100B.
During the secondary phase of TBI, inflammation-induced apoptosis facilitates the remodeling of brain tissue. Both pathways for apoptosis are initiated by CASP3 and increased levels are significantly correlated with lower GCS and higher mortality [20]. TBARS, the most widely used assay for indexing lipid peroxidation end product malondialdehyde is also valuable in evaluating oxidative stress damage of lipids and proteins. Previously published results reported increased TBARS levels in patients after TBI with lower GCS scores and higher mortality rates [21].
SUM group had lower levels of S100B, CASP3 and TBARS compared with the trauma group and similar levels when compared with the control group. Due to a small sample size and a high standard deviation, only TBARS comparison yielded statistically significant results, but these results do shed hope for further studies with an increased number of subjects. Nevertheless, the mechanism by which SUM exerts these effects needs to be delineated.
A well-designed experimental study evaluating the effect of SUM on PTH showed that SUM was found to decrease inducible nitric oxide synthase (iNOS) and CGRP both known to increase after closed cortical injury [12]. iNOS and CGRP are induced by pro-inflammatory cytokines such as TNF-a, IL-1b, and IL-6 after TBI [33]. Nitric oxide (NO) is believed to increase immediately after trauma and several days after. Reducing or inhibiting the release of NO after TBI has yielded improved neurological outcome along with neuroprotective effects [34]. SUM in this study may have decreased NOS levels, thus reducing the inflammatory response by creating a better neurological outcome confirmed by the analyses.
A study evaluating the role of transient receptor potential vanilloid 1 (TRPV1) channel in PTH found that SUM administration was effective in reducing periorbital allodynia. The authors have concluded that TRPV1 induced neuroinflammation after TBI. TRPV1 collaborated with CGRP in activating astrocytes and microglia leading to the increased inflammation and vasodilatation. SUM was able to reduce allodynia via blocking CGRP release leading to decreased inflammation evidenced by a decrease in GFAP [13].
The aforementioned studies involving PTH are essential because PTH is mainly caused by the neuroinflammatory process of TBI, hence any study evaluating a pharmacological agent for the treatment for PTH would indirectly be treating the inflammatory process. However, these comprehensive studies are valuable in elucidating the most probable mechanism in which SUM is able to impose an anti-inflammatory effect. The findings support the analyses of this study as well. Inflammatory markers evaluated in this study were found to be decreased by SUM, which was further supported by histological examination and neurological assessment.
Histopathological evaluation provides valuable insight into the micro-analysis of cellular structure of the tissues along with the extent of tissue deterioration. A novel scoring system evaluating four different parameters was used to confirm biochemical and neurological assessment, which was previously used in other studies. This scoring system may become a valuable parameter for future studies. In this paper, SUM receiving subjects had statistically significantly higher anti-inflammatory and neuroprotective scores.
Consequently, in order to assess the neurological outcomes of the subjects, the Garcia Test was used in this study, which evaluates sensorimotor deficits. Biochemical and histological evaluations may provide results that do not correlate well with final results, evaluated through neurological examination. Thus, the Garcia Test provides a valuable assessment of the agent used, in this case SUM, which provided statistically significant results with higher scores compared to the trauma group.
Experimental TBI and agents to counteract the secondary neuro-inflammation phase are being continuously evaluated as they continue to be a popular topic. Neuroscientists are aiming to go just beyond mannitol or hypertonic saline treatment after TBI [35] or supportive treatment such as blood transfusions [36]. Modifying cation homeostasis to prevent the influx of Ca2+ and Na+ and glutamate antagonists to prevent excitatory effects, and caspase inhibition have also been a popular target [37]. A summary of agents that have been previously evaluated is shown in Tab. 1.
Conclusion
Numerous agents have been and are currently being investigated to reverse the effects of the secondary phase after trauma, but very few have made it into clinical use. This paper evaluated SUM’s effects in experimental TBI, comprising neurological, histological and biochemical parameters. The histopathological assessments used in this paper have been used in previous studies, but to our knowledge a scoring system validating biochemical results and neurological assessments is the first one. The results of this study provide valuable insight regarding the effects of a readily accessible and affordable migraine drug on a very frequent clinical entity. However, the low number of subjects has yielded some statistically insignificant results. SUM may prove to be a valuable agent in decreasing brain injury and functional deficits after TBI.
Ethical principles
All experimental procedures were performed in compliance with the “Principles of Laboratory Animal Care” (NIH publication 82‑23, revised in 1985 and further implemented in 1996) and were approved by Ankara Research and Training Hospital Ethics Committee (date: 24. 5. 2016; no: 0032/240516/p10). ARRIVE guidelines were strictly followed throughout the experiment.
Acknowledgements
The authors would like to thank Mr. Ahmet Ekinci for his graphical construct of the sampled areas of the specimens.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Ismail Bozkurt
Clinic of Neurosurgery
Cankiri State Hospital
Kırkevler, Kastamonu Cd. No:236
18100 Cankiri
Turkey
e-mail: ibozkurt85@gmail.com
Accepted for review: 28. 1. 2022
Accepted for print: 8. 9. 2022
Sources
1. Lee HF, Lin JS, Chang CF. Acute Kahweol treatment attenuates traumatic brain injury neuroinflammation and functional deficits. Nutrients 2019; 11 (10): 2301. doi: 10.3390/nu11102301.
2. Gyoneva S, Ransohoff RM. Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol Sci 2015; 36 (7): 471–480. doi: 10.1016/ j.tips.2015.04.003.
3. Morganti-Kossmann MC, Semple BD, Hellewell SC et al. The complexity of neuroinflammation consequent to traumatic brain injury: from research evidence to potential treatments. Acta Neuropathol 2019; 137 (5): 731–755. doi: 10.1007/s00401-018-1944-6.
4. Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol 2015; 127: 3–13. doi: 10.1016/B978-0-444-52892-6.00001-5.
5. Chen G, Shi JX, Hang CH et al. Inhibitory effect on cerebral inflammatory agents that accompany traumatic brain injury in a rat model: a potential neuroprotective mechanism of recombinant human erythropoietin (rhEPO). Neurosci Lett 2007; 425 (3): 177–182. doi: 10.1016/j.neulet.2007.08.022.
6. DeWitt DS, Hawkins BE, Dixon CE et al. Pre-clinical testing of therapies for traumatic brain injury. J Neurotrauma 2018; 35 (23): 2737–2754. doi: 10.1089/neu.2018.5778.
7. Khalilzadeh M, Panahi G, Rashidian A et al. The protective effects of sumatriptan on vincristine – induced peripheral neuropathy in a rat model. Neurotoxicology 2018; 67: 279–286. doi: 10.1016/j.neuro.2018.06.012.
8. Dejban P, Rahimi N, Takzare N et al. Protective effects of sumatriptan on ischaemia/reperfusion injury following torsion/detorsion in ipsilateral and contralateral testes of rat. Andrologia 2019; 51 (9): e13358. doi: 10.1111/and.13358.
9. Mies G. Neuroprotective effect of sumatriptan, a 5-HT (1D) receptor agonist, in focal cerebral ischemia of rat brain. J Stroke Cerebrovasc Dis 1998; 7 (4): 242–249. doi: 10.1016/s1052-3057 (98) 80033-5.
10. Sheibani M, Faghir-Ghanesefat H, Dehpour S et al. Sumatriptan protects against myocardial ischaemia-reperfusion injury by inhibition of inflammation in rat model. Inflammopharmacology 2019; 27 (5): 1071–1080. doi: 10.1007/s10787-019-00586-5.
11. Dehdashtian A, Afshari K, Zarifeh Jazaeri S et al. Sumatriptan increases skin flap survival through activation of 5-hydroxytryptamine 1b/1d receptors in rats: the mediating role of the nitric oxide pathway. Plast Reconstr Surg 2019; 144 (1): 70e–77e. doi: 10.1097/PRS.00000 00000005740.
12. Daiutolo BV, Tyburski A, Clark SW et al. Trigeminal pain molecules, allodynia, and photosensitivity are pharmacologically and genetically modulated in a model of traumatic brain injury. J Neurotrauma 2016; 33 (8): 748–760. doi: 10.1089/neu.2015.4087.
13. da Silva Fiorin F, do Espírito Santo CC, do Nascimento RS et al. Capsaicin-sensitive fibers mediate periorbital allodynia and activation of inflammatory cells after traumatic brain injury in rats: involvement of TRPV1 channels in post-traumatic headache. Neuropharmacology 2020; 176: 108215. doi: 10.1016/j.neuropharm.2020.108215.
14. Yue JK, Burke JF, Upadhyayula PS et al. Selective serotonin reuptake inhibitors for treating neurocognitive and neuropsychiatric disorders following traumatic brain injury: an evaluation of current evidence. Brain Sci 2017; 7 (8): 93. doi: 10.3390/brainsci7080093.
15. Li Q, Wang P, Huang C et al. N-acetyl serotonin protects neural progenitor cells against oxidative stress-induced apoptosis and improves neurogenesis in adult mouse hippocampus following traumatic brain injury. J Mol Neurosci 2019; 67 (4): 574–588. doi: 10.1007/s12031-019-01263-6.
16. Marmarou A, Foda MA, van den Brink W et al. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg 1994; 80 (2): 291–300. doi: 10.3171/jns.1994.80.2.0291.
17. Garcia JH, Wagner S, Liu KF et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995; 26 (4): 627–635. doi: 10.1161/01.str.26.4.627.
18. Ross SA, Cunningham RT, Johnston CF et al. Neuron-specific enolase as an aid to outcome prediction in head injury. Br J Neurosurg 1996; 10 (5): 471–476. doi: 10.1080/02688699647104.
19. Hårdemark HG, Ericsson N, Kotwica Z et al. S-100 protein and neuron-specific enolase in CSF after experimental traumatic or focal ischemic brain damage. J Neurosurg 1989; 71 (5 Pt 1): 727–731. doi: 10.3171/jns.1989.71.5.0727.
20. Lorente L, Martín MM, Argueso M et al. Serum caspase-3 levels and mortality are associated in patients with severe traumatic brain injury. BMC Neurol 2015; 15: 228. doi: 10.1186/s12883-015-0485-z.
21. Hohl A, Gullo Jda S, Silva CC et al. Plasma levels of oxidative stress biomarkers and hospital mortality in severe head injury: a multivariate analysis. J Crit Care 2012; 27 (5): 523.e11–523.e5.23e19. doi: 10.1016/j.jcrc.2011.06.007.
22. Carmichael NM, Charlton MP, Dostrovsky JO. Activation of the 5-HT1B/D receptor reduces hindlimb neurogenic inflammation caused by sensory nerve stimulation and capsaicin. Pain 2008; 134 (1–2): 97–105. doi: 10.1016/j.pain.2007.03.037.
23. Mobasheran P, Rajai N, Kohansal P et al. The effects of acute sumatriptan treatment on renal ischemia/reperfusion injury in rat and the possible involvement of nitric oxide. Can J Physiol Pharmacol 2020; 98 (4): 252–258. doi: 10.1139/cjpp-2019-0301.
24. Pfaffenrath V, Cunin G, Sjonell G et al. Efficacy and safety of sumatriptan tablets (25 mg, 50 mg, and 100 mg) in the acute treatment of migraine: defining the optimum doses of oral sumatriptan. Headache 1998; 38 (3): 184–190. doi: 10.1046/j.1526-4610.1998.3803184.x.
25. Humphrey PP, Goadsby PJ. The mode of action of sumatriptan is vascular? A debate. Cephalalgia 1994; 14 (6): 401–393. doi: 10.1046/j.1468-2982.1994.1406401.x.
26. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci 2013; 14 (2): 128–142. doi: 10.1038/nrn3407.
27. Herman ST. Acute seizures and status epilepticus. In: Skolnick BE, Alves WM (eds). Handbook of neuroemergency clinical trials. San Diego: Academic Press 2018: 189–230.
28. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol 2015; 867: 125–143. doi: 10.1007/978-94-017-7215-0_9.
29. Matute-Blanch C, Montalban X, Comabella M. Multiple sclerosis, and other demyelinating and autoimmune inflammatory diseases of the central nervous system. Handb Clin Neurol 2017; 146: 67–84. doi: 10.1016/B978-0-12-804279-3.00005-8.
30. Helbok R, Beer R. Cerebrospinal fluid and brain extracellular fluid in severe brain trauma. Handb Clin Neurol 2017; 146: 237–258. doi: 10.1016/B978-0-12-804279-3.00014-9.
31. Park SH, Hwang SK. Prognostic value of serum levels of S100 calcium-binding protein B, neuron-specific enolase, and interleukin-6 in pediatric patients with traumatic brain injury. World Neurosurg 2018; 118: e534–e542. doi: 10.1016/j.wneu.2018.06.234.
32. Donato R, Cannon BR, Sorci G et al. Functions of S100 proteins. Curr Mol Med 2013; 13 (1): 24–57.
33. Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol 1998; 113 (2): 147–156. doi: 10.1046/j.1365-2249.1998.00648.x.
34. Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol 2004; 14 (2): 195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x.
35. Deora H, Shukla D. Sugar or salt? Survey on the use of mannitol or hypertonic saline for cerebral edema due to traumatic brain injury. Neurol India 2021; 69 (1): 212–213. doi: 10.4103/0028-3886.310111.
36. Florez-Perdomo WA, García-Ballestas E, Martinez-Perez R et al. Hemoglobin levels as a transfusion criterion in moderate to severe traumatic brain injury: a systematic review and meta-analysis. Br J Neurosurg 2021; 21: 1–7. doi: 10.1080/02688697.2021.1940850.
37. Laurer HL, McIntosh TK. Pharmacologic therapy in traumatic brain injury: update on experimental treatment strategies. Curr Pharm Des 2001; 7 (15): 1505–1516. doi: 10.2174/1381612013397285.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2022 Issue 5
Most read in this issue
- Lumbar spine disorder – the new occupational disease
- Cenobamate
- Carotid web
- The dentate gyrus – anatomy, vascular supply, function and neuropathology


