Surgical management of foramen magnum tumours – experiences with 20 cases
Chirurgická léčba nádorů v oblasti foramen magnum – zkušenosti ze 20 případů
Cíle: Nádory v oblasti foramen magnum představují přibližně 1,1–3,8 % nádorů CNS. Cílem této studie bylo vyhodnotit výsledky mikrochirurgické léčby nádorů v oblasti foramen magnum v rámci retrospektivní analýzy 20 případů, které byly operovány mezi roky 2007 a 2020. Materiály a metody: Byly analyzovány věk v době diagnózy, pohlaví, umístění a velikost nádoru v sagitální rovině, chirurgický přístup, rozsah resekce, příznaky při příjmu a po chirurgickém zákroku, trvání příznaků a délka hospitalizace od zákroku po propuštění. Výsledky: Z pacientů bylo 15 žen a 5 mužů. Průměrný věk v době diagnózy byl 55 let. Jednalo se většinou o extraspinální a intradurální nádory o velikosti 10–30 mm v sagitální rovině na zobrazení MR. Nejčastěji prováděným zákrokem byla hemilaminektomie z posterolaterálního přístupu. K úplné resekci došlo přibližně v 85 % případů. Mezi nejčastějšími příznaky byly bolest hlavy oslabení s trváním delším než jeden rok. 90 % pacientů uvedlo zlepšení klinického stavu. Délka hospitalizace od chirurgického zákroku do propuštění byla přibližně 12 dní. Závěr: I přes anatomicky obtížně přístupnou oblast je u pacientů s nádorem v oblasti foramen magnum možné provést mikrochirurgické operace bezpečným způsobem.
Klíčová slova:
foramen magnum – meningiom – mícha – neurinóm
Authors:
M. Chwiałkowski; A. Koziarski; G. Zieliński
Authors‘ workplace:
Institute of Medicine, Warsaw, Poland
; Department of Neurosurgery, Military
Published in:
Cesk Slov Neurol N 2021; 84/117(2): 188-194
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn2021188
Overview
Aim: Foramen magnum tumours represent about 1.1–3.8% of all CNS tumours. The aim of this study was to evaluate the results of the microsurgical treatment of foramen magnum tumours with a retrospective analysis of 20 cases operated upon between 2007 and 2020. Material and Methods: Age at diagnosis, sex, location and size of the tumour in the sagittal plane, surgical approach, extent of resection, symptoms upon admission and after surgery, the duration of the symptoms and length of hospitalization time from surgery until discharge were analyzed. Results: 15 patients were women and 5 were men. The average age at diagnosis was 55 years. There were mostly extraspinal and intradural tumours between 1030 mm in size in the sagittal dimension in the MRI examination. The most common procedure performed was hemilaminectomy with a posterolateral approach. Total resection rate was about 85%. The most common symptoms were headache and weakness with duration of more than a year. 90% of patients reported improvement of their clinical status. The hospitalization length from surgery until discharge was about 12 days. Conclusion: Despite the anatomically difficult area, microsurgical operations can be performed in a safe manner in patients suffering from a foramen magnum tumour.
Keywords:
foramen magnum – Meningioma – Spinal cord – neurinoma
Background
Foramen magnum tumours represent about 1.1–3.8% of all CNS tumours [1]. They were first described as incidental findings by the French physician Hallopeau in 1872 in a post-mortem study. The first successful operation of tumours in the region dates back to 1927 [2–4]. Treatment of the lesions located in this region is challenging because of the vicinity of crucial anatomical structures including medulla, lower group of cranial nerves and vertebral artery [2]. The most common foramen magnum tumours are meningiomas (1.8–3.2% of all meningiomas) followed by neurinomas. Together they constitute up to 40.5–75.0% of the tumours located in this region. Other tumour types located in this region include chordomas, chondroids, metastases and epidermal cysts [1,5–8].
The aim of this study was to evaluate the results of the microsurgical treatment of foramen magnum tumours treated.
Material and methods
The medical history of 20 patients who underwent surgery for a tumour located in the foramen magnum region between the years 2007 and 2020 at the Neurosurgery Department of the Military Institute of Medicine in Warsaw, Poland was analyzed retrospectively. We observed the age at diagnosis and which sex was more susceptible to this type of tumour. Other analyzed factors were the location in relation to the neuraxis and the size of the tumour in the sagittal plane in MRI T2- and T1-weighted images, especially with regard to neurovascular structures which could determine the surgical approach and extent of the resection. We studied the symptoms of foramen magnum tumours upon admission and after the procedure and also, the duration of the symptoms and the average hospitalization lenght.
In our department, neuromonitoring during surgery for extra-spinal tumours is not generally used.
Results
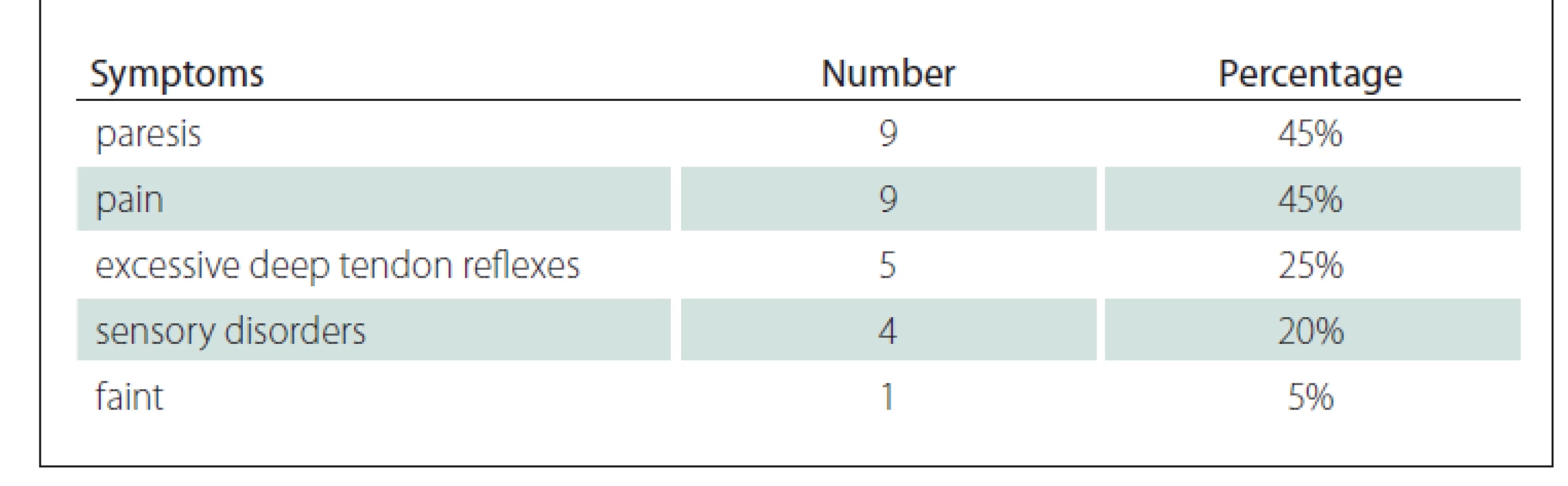
Medical history of 20 patients was analyzed. 15 (75%) patients were women and 5 (25%) patients were men. The female/ male ratio was 3 : 1. The average age at diagnosis was 55 years. Patients presented with two types of clinical course: long-lasting chronic symptoms and more rapid development of symptoms lasting less than one year. The shortest course was about 3 months.
The main symptoms upon admission were motor dysfunction and headache. Other neurological disorders included sensory disturbances, excessive deep tendon reflexes and one case of periodic fainting. The symptoms are summarized in (Tab. 1).
The best method for visualizing spinal canal lesions is MRI [9]. Each tumour was routinely assessed using this modality before and after surgery. There were 18 extramedullar intradural tumours and 2 epidural tumours. The location of the tumour was also determined based on the MRI examination in relation to the neuroaxis. In 2 cases the tumour was posterior to the spinal cord, in 6 cases it was anterior and in the remaining 12 cases the tumour was located anterolaterally to the spinal cord.
Tumours were measured in the sagittal dimensions and were divided according to their size into three groups based on measurements performed on the MRI scans: group I: < 10 mm; group II: 10–30 mm; group III: > 30 mm. Tumours size range in the sagittal dimensions was 8–53 mm. The majority of the tumours were in the range of 10–30 mm in the sagittal dimensions (Fig. 1).
Obr. 1. Velikost nádorů.

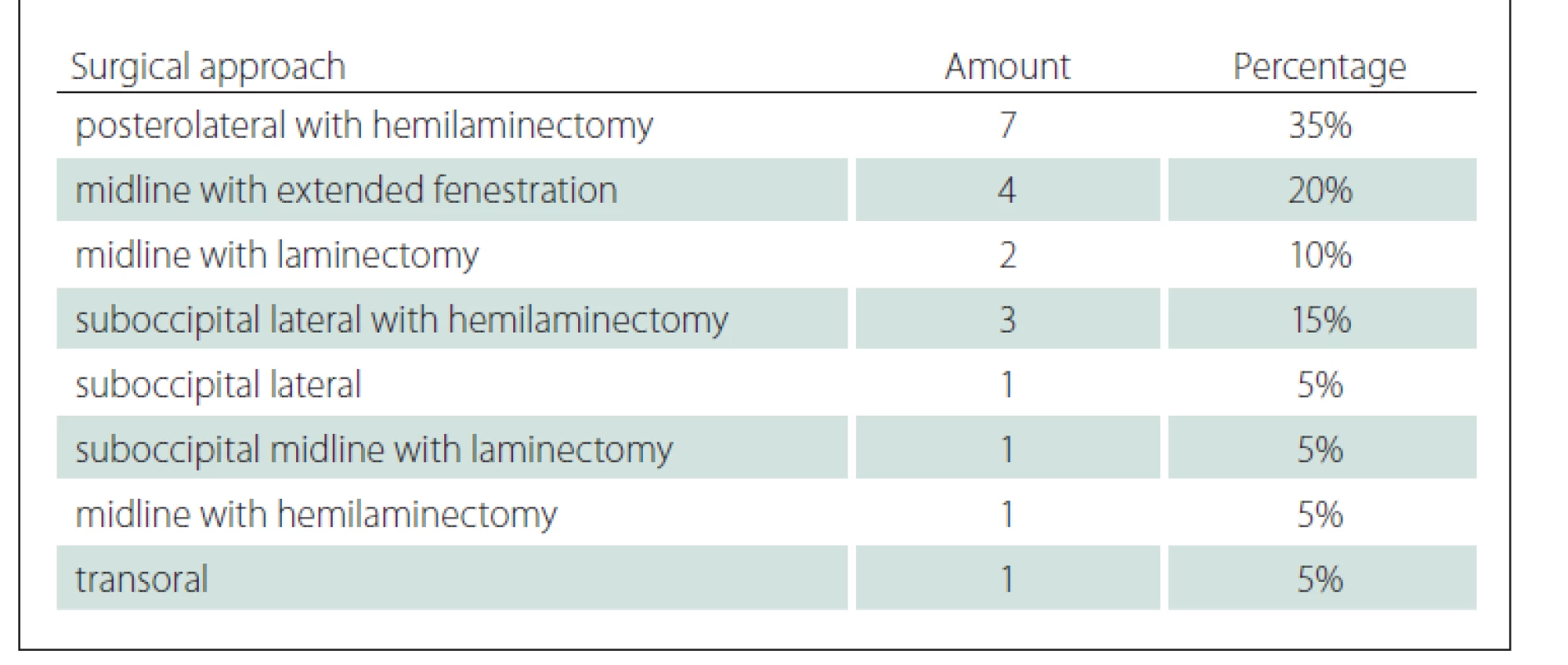
Several surgical techniques were used. These were hemilaminectomy from the postero-lateral approach (7 patients) and midline approach (1 patient), midline approach laminectomy (2 patients), lateral suboccipital craniotomy (in 4 patients, including one extended by right-sided hemilaminectomy and two by left-sided hemilamectomy) and 1 subboccipital midline approach (extended with laminectomy in one patient), 4 midline approaches with extended fenestrations and 1 transoral approach (Fig. 2, Tab. 2).
Obr. 2. Obří chordom v oblasti foramen magnum operovaný transorálním přístupem –
dosaženo celkové resekce.

In 17 (85%) patients, the tumour was macroscopically removed completely while in 3 (15%) patients the removal was incomplete. The histopathological examination revealed 12 meningiomas, 6 neurinomas, 1 chordoma and 1 enterogenic cyst.
Eighteen patients (90%) reported improvement of their preoperative symptoms. Two (10%) patients developed post-operative complications: lesion of the XIIth and XIth cranial nerve (1 patient) and quadriplegia (1 patient).
The average time from surgery to discharge was 12 days. In one case, the postoperative course was severe as the patient developed quadriplegia and required assisted ventilation and nutritional alimentation. Intensive rehabilitation was implemented and patient recovered from her symptoms and now she is self-sufficient. The hospitalization time was 72 days. Notwithstanding this challenging case, the length hospitalization would be 8.7 days.
Discussion
Foramen magnum region is the space bounded anteriorly by the lower part of the clivus and the upper edge of the body of the C2 vertebra and posteriorly by the front edge of the occipital bone and the C2 spinous process. The lateral borders are composed of the jugular tubercules and the upper part of the C2 arch and the C1–C2 facet joints [8,10,11]. Cushing and Eisenhardt divided tumours of the foramen magnum into supraforaminal/ craniospinal (i.e., those that form on the base of the skull) and subforaminal/ spinocranial (arising in the spinal canal) groups [12,13]. They are characterized by an ambiguous clinical course. The symptoms can even resemble the features of cervical spine degenerative disease [1]. Surgery is the main treatment modality for most of the tumours located in this region with the main aim being to decompress the medulla and the spinal cord with minimal damage to the neural tissue. Various surgical approaches have been proposed over the years: suboccipital midline approach, lateral with or without partial resection of the occipital condyle, transposition of the vertebral artery, far lateral, anterior and anterolateral, access through the oral cavity and many others [2,6,8,10,14,15]. The choice of the approach is largely influenced by the location of the tumour relative to the neuroaxis. In our department, the most commonly used approaches are the posterior midline and posterolateral approach. These approaches have been reported previously by several authors [5,10–12,14,15]. In our experience, with adequate exposure and utilizing the posterolateral approach, time consuming and risky manoeuvres that are reported in the literature such as transposition of the vertebral artery or resection of the occipital condyles can be avoided even in large tumours localized ventral to the medulla (Fig. 3) [16].
Obr. 3. Případ meningiomu umístěného anteriorně k ose nervového systému operovaného
subokcipitálním laterálním přístupem s hemilaminektomií C1. Bylo dosaženo celkové
resekce bez resekce okcipitálního kondylu nebo transpozice a. vertebralis. Snímky
před zákrokem (a, c) a po zákroku (b, d).

The most effective approach in the case of craniospinal junction tumours extending into the posterior cranial fossa is the suboccipital lateral approach [5,6,17,18]. The encasement of the posterior inferior cerebellar artery and lower group of cranial nerves can prevent gross total resection (GTR) of these tumours (Fig. 4).
Obr. 4 (a–d). Případ meningiomu umístěného
anterolaterálně vzhledem k ose nervového
systému obalujícího a. cerebelli
posterior inferior a XII. hlavový nerv operovaného
pomocí subokcipitální laterální
kraniotomie – neúplné odstranění. Snímky
před zákrokem a po zákroku. (e–j) Případ
meningiomu umístěného anteriorně vzhledem
k ose nervového systému obalujícího
dolní skupinu hlavových nervů operovaného
pomocí subokcipitálního laterálního
přístupu s hemilaminektomií C1 – neúplné
odstranění. Snímky před zákrokem a po zákroku
a dále z průběhu operace.

In the paper by Choque-Velasquez and Hernesniemi, we can find a similar approach to tumours of this type without resection of the occipital condyle and transposition of the vertebral artery [17]. An approach with the caudal extension of the tumour hemilaminectomy (C1/ C2 – 10 cases in our group) or laminectomy (C1/ C2 – 3 cases) can be employed (Fig. 5).
(a, b) Preoperative axial and sagittal T2-weinhted MRI image.
(c, d) Axial and sagittal postoperative T2-weighted MRI image.
(e, f) Intraoperative image of meningioma with fi bre of C2.
Obr. 5. Meningeom umístěný anterolaterálně k ose nervového systému operovaný hemilaminektomií C1 – úplné odstranění.
Snímky před zákrokem a po zákroku.
(a, b) Axiální a sagitální T2 vážené MR snímky před operací.
(c, d) Axiální a sagitální T2 vážené MR snímky po operaci.
(e, f) Intraoperačvní snímek meningiomu s vlákny C2.

Laminectomy used to be the standard approach for spinal canal tumours. Currently, it is used mainly in the case of changes located posteriorly to the spinal cord [19].
On the other hand, unilateral hemilaminectomy became more popular as a minimally invasive method in the surgery for intradural tumours with a lateral position in relation to the spinal cord [19]. The best conditions for its implementation mentioned in the literature are the transverse size of the tumour < 2 cm and the tumour limitation to 2 levels of the spinal canal [20,21]. Our material proves that hemilaminectomy can be used successfully for resection of these kinds of tumours.
It is difficult to achieve GTR in tumours > 2 cm in diameter or exceeding 2 levels using only hemilaminectomy. For the most part, we can extend our procedure (i.e., suboccipital lateral craniotomy with hemilaminectomy) to remove these kinds of tumours. In our material, laminectomy was performed in three cases and in one case a suboccipital craniotomy was extended with laminectomy in tumours located on the dorsal surface of the spinal cord. In four cases a tumour removal was possible through a widened fenestration. In the analyzed literature, we did not encounter any cases of foramen magnum tumour removal through a fenestration alone. Similarly to Bernard Georg et al, we use a seated position. It has many advantages, including facilitating the drainage of blood and cerebrospinal fluid from the operating field and rapid decompression of the nerve structures. The frequently quoted disadvantage of this approach is the air embolism [15]. In our material, with implementation of standard surgical manoeuvres, no significant intraoperative air embolism occurred. These manoeuvres are executed as soon as the fall of the end-tidal CO2 level is observed and include generous irrigation, compression of the jugular veins, increase of the venous pressure by tilting the whole operative table head-down, waxing the bony edges and covering the sinuses with moist gelfoam.
Using the above-mentioned techniques, we achieved GTR in 85% of 20 patients. For comparison, the rate of GTR in the material of C. Parlato was 73.3% of 15 patients and in the Kuntal Kanti Das case series it was 51.7% of 29 patients. In 2 cases, due to the large size of the tumour and its adhesions with the surrounding structures, the course of the posterior inferior cerebellar artery and the lower group of cranial nerves, we failed to achieve GTR (Fig. 4, 6). In addition, the ventral position of the tumour in relation to the spinal cord has a significant influence on the total tumour resection, which was the cause of failure to achieve GTR in 2 cases in our set [18]. Among the 4 incompletely removed tumours, two of them were in group III in the sagittal dimension according to the tumour size but on the other hand, 5 were in the same group with GTR. There were no mortalities.
Obr. 6. Obří neurom umístěný anteriorně a anterolaterálně vzhledem k ose nervového
systému s nepříznivým průběhem a. vertebralis, a. cerebelli posterior inferior a XII.
a XI. hlavového nervu v blízkosti (operováno přístupem ve střední linii s laminektomií C1
a hemilaminektomií C2).

Similarly to Parlato, there was a female predominance (women/ men ratio 3 : 1) [14]. We also obtained similar results with the above-mentioned author by analyzing the average age at onset (55 vs. 52 years). However, Kuntal Kanti Das’s results are different. Here, the average age at onset was 36.6 years with a predominance of men. The most frequent symptoms of these types of changes were muscle weakness and pain in 45% of patients in our work; disorder of muscular strength in 75% of Kanti Das’s work; and pain complaints from 46% of patients in Parlato’s work. Most of the tumours of this region were meningiomas and neuromas – 12 and 6, respectively. Spinal canal meningiomas constitute 12% of all tumours and as much as 25–45% of intradural spinal canal tumours [1,7,8]. In Parlato’s publication, histopathology results also showed the dominance of these tumours – 7 and 5, respectively. In Kanti Das’s work, it was just the opposite – 11 neuromas and 5 meningiomas. In 90% of the patients who underwent surgery, we obtained neurological improvement. Only two patients developed complications such as the lesions of cranial nerves XII and XI in the first and limb paresis in the second case. Compared to the work of Parlato (complications in 2 out of 15 patients – 14%) and Kanti Das (6 out of 29 patients – 21%) in our set, the percentage of complications was about 10% [10,14].
Conclusions
Despite the anatomically difficult area, microsurgical operations can be performed in a safe manner in patients suffering from a foramen magnum tumour. We did not use neuronavigation or intraoperative neuromonitoring and in comparison with other centres the final results of the treatment were similar [15,20].
Both posterior and posterolateral approaches and a seated position, as seen in the analyzed patient set, are safe methods of microsurgical surgery of foramen magnum tumours. The most important factors that may affect the postoperative course are tumour location and adhesions to surrounding vascular and neural structures [22].
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). For this type of study, formal consent was not required.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Miłosz Chwiałkowski, MD
Department of Neurosurgery
Military Institute of Medicine
Szaserów 128 street
04-141 Warsaw
Poland
e-mail: milosz.chwialkowski@gmail.com
Sources
1. Greenberg MS. Handbook of neurosurgery. 8th ed. New York: Thieme 2016: 1367–1368.
2. Ariza D, Campera A, Chaparro R et al. Key aspects in foramen magnum meningiomas: from old neuroanatomical conceptions to current far lateral neurosurgucal intervention. World Neurosurgy 2017; 106; 477–483. doi: 10.1016/ j.wneu.2017.07.029.
3. Elsberg CA, Strauss I. Tumors of the spinal cord which project into the posterior cranial fossa. Report of a case in which a growth was removed from ventral and lateral aspects of the medulla oblongata and upper cervical cord. Arch Neurol Psychiatry 1929; 21(2): 261–273. doi: 10.1001/ archneurpsyc.1929.02210200017003.
4. Hallopeau H. Note about two cases of tumors of the mesencephalon. Gaz Med Paris 1874; 3: 2.
5. Goeal A, Desai K, Muzumdar D. Surgery on anterior foramen magnum meningiomas using a conventional posterior suboccipital approach: a case report on an experience with 17 cases. Neurosugery 2001; 49(1): 102–106. doi: 10.1097/ 00006123-200107000-00016.
6. Kumar A, Bhaskar S, Bhardwaj M et al. Foramen magnum chordoid meningioma in a 22-year-old female. Asian J Neurosurg 2018; 13(3); 834–837. doi: 10.4103/ ajns.AJNS_296_16.
7. Nakamura M, Tsuji O, Fujiyoshi K et al. Long-term surgical outcomes of spinal meningiomas. Spine 2012; 37(10): 617–623. doi: 10.1097/ BRS.0b013e31824167f1.
8. Ramina R, Landeiro JA, Acioly MA et al. Samii’s essential in neurosurgery, tumors of the craniocervical junction: overview and update. New York: Springer 2014: 417–430.
9. Hu L, Wang Ch. Intramedullary melanotic schwannoma of the cervical spine: a case report and literature review. Mol Clin Oncol 2018; 8(4): 567–570. doi: 10.3892/ mco.2018.1584.
10. Das KK, Kumar R, Ashish K et al. Extramedullary foramen magnum tumors and their surgical management: an Experience with 29 cases. Asian J Neurosurg 2014; 9(4): 223–232. doi: 10.4103/ 1793-5482.146616.
11. George B, Lot G, Boissonnet H. Meningioma of the foramen magnum: a series of 40 cases; Surg Neurol 1997; 47(4): 371–379. doi: 10.1016/ s0090-3019(96)00204-2.
12. Bydon M, Ma TM, Risheng X et al. Surgical outcomes of craniocervical junction meningiomas: a series of 22 consecutive patients. Clin Neurol Neurosurg 2014; 117: 71–79. doi: 10.1016/ j.clineuro.2013.11.023.
13. Cushing H, Eisenhardt L. Meningiomas: their classification, regional behaviour, life history and surgical end results. Bull Med Libr Assoc 1938; 27(2): 185.
14. Parlato C, Tessitore E, Schonauer E et al. Management of bening craniocervical junction tumors. ActaNeurochir 2003; 145(1); 31–36. doi: 10.1007/ s00701-002-1024-4.
15. Wu Z, Hao S, Zhang J et al. Foramen magnum meningiomas: experiences in 114 patients at a single institute over 15 years. Surg Neurol 2009; 72(4): 376–382. doi: 10.1016/ j.surneu.2009.05.006.
16. Park H, Lee KS, Hong CK. Vertebral artery transposition via an extreme-lateral approach for anterior foramen magnum meningiom or craniocervical junction tumors. World Neurosurg 2016; 88: 154–165. doi: 10.1016/ j.wneu.2015.12.073.
17. Choque-Velasquez J, Hernesniemi J. One burr-hole craniotomy: enough lateral approach to foramen magnum in Helsinki Neurosurgery. Surg Neurol Int 2018; 9: 165. doi: 10.4103/ sni.sni_193_18.
18. Sami M, Klekamp J, Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery 1996; 39(6): 1086–1094. doi: 10.1097/ 00006123-199612000-00003.
19. Notani N, Miyazaki M, Kanezaki S et al. Surgical management of ventrally located spinal meningiomas via posteriori approach. Eur J Orthop Surg Traumatol 2017; 27(2): 181–186. doi: 10.1007/ s00590-016-1860-1.
20. Kutty RK, Sreemathyamma SB, Sivanandapanicker JL et al. Hemilaminectomy for spinal cord intradural tumors: an institutional experience. Asian J Neurosurg 2018; 13(3): 760–765. doi: 10.4103/ ajns.AJNS_106_18.
21. Villalonga JF, Cervio A. Surgical treatment of intradural extramedullary lesions by hemilaminectomy. Surg Neurol Int 2017; 8 (Suppl 2): S11–S17. doi: 10.4103/ sni.sni_253_17.
22. Campera A, Ajler P, Roman G et al. Foramen magnum meningiomas: a report of 12 cases and literature review. Surg Neurol Int 2017; 8 (Suppl 2): S25–S36. doi: 10.4103/ sni.sni_277_17.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2021 Issue 2
Most read in this issue
- Morton’s neuralgia, metatarsalgia
- Moyamoya disease
- Correct and incorrect naming of pictures for the more demanding written Picture Naming and Immediate Recall test (door PICNIR)
- Etiopathogenesis and diagnostics of progressive multifocal leukoencephalopathy in patients treated with natalizumab