High-sensitive CRP in ischaemic stroke patients – from risk factors to evolution
CRP stanovený vysoce senzitivní metodou u pacientů s ichemickou cévní mozkovou příhodou – od rizikových faktorů po vývoj
Cíl: Bylo prokázáno, že C-reaktivní protein stanovený vysoce senzitivní metodou (high-sensitive C-reactive protein; hs-CRP) má dobrou senzitivitu při diagnostice cerebrovaskulárních příhod. Cílem studie bylo vyhodnotit spojitosti mezi hs-CRP a rizikovými faktory CMP, neurologickou poruchou, ischemií mozku a prognózou.
Materiál a metody: Studie zahrnula 153 pacientů rozdělených do dvou skupin: pacienti s akutní ischemickou CMP a pacienti s rizikovými faktory, kteří CMP neprodělali. V odebraných vzorcích krve byla stanovena hladina hs-CRP v séru. Neurologické postižení bylo hodnoceno při přijetí do nemocnice a při propuštění pomocí NIHSS (National Institute of Health Score Scale) škály. Ve stejné skupině pacientů bylo v průběhu prvních 24 h po rozvinutí CMP provedeno vyšetření CT a byly zaznamenány známky časných ischemických lézí. Případy CMP byly rozděleny do podskupin pomocí TOAST klasifikace.
Výsledky: Hladiny hs-CRP v séru pacientů s CMP byly významně vyšší v porovnání s pacienty, kteří CMP neměli. Nejvyšší podíl pacientů s hladinami hs-CRP > 3 mg/ l byl pozorován u pacientů s CMP nejasné etiologie. U hs-CRP byla ukázána významná diagnostická schopnost identifikovat CMP; optimálním kritériem byla hladina 4,29 mg/ l. Poměr šancí u hs-CRP pozitivních pacientů ukázal zvýšené riziko CMP a ischemických lézí. Hladina hs-CRP pozitivně korelovala s NIHSS a časem testování a negativně s vývojem CMP.
Závěr: Hs-CRP je spolehlivý marker, který může být nápomocen při diagnóze CMP, její etiologické klasifikaci, klinických projevech a prognóze. Naše výsledky přispívají k identifikaci charakteristik CMP v populaci Bulharska.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Klíčová slova:
high-sensitive C-reactive protein – stroke biomarkers – acute ischaemic stroke
Authors:
M. Peycheva 1; T. Deneva 2; Z. Zahariev 1
Authors‘ workplace:
Department of Neurology, Medical, University Plovdiv, Bulgaria
1; Department of Clinical Laboratory, Medical University Plovdiv, Bulgaria
2
Published in:
Cesk Slov Neurol N 2019; 82(5): 549-555
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn2019549
Overview
Aim: High-sensitive C-reactive protein (hs-CRP) has demonstrated good sensitivity in the diagnosis of cerebrovascular events. The study aimed to explore the associations between hs-CRP and stroke risk factors, neurological impairment, cerebral ischaemia and prognosis.
Materials and methods: The study involved 153 patients categorized into two groups: patients with acute ischaemic stroke and patients with risk factors who had not had a stroke. Blood samples were collected to analyze the serum level of hs-CRP. The National Institutes of Health Stroke Scale (NIHSS) was used to determine the neurological disability of the stroke patients upon hospital admission and upon discharge. Cerebral CT was performed on the same group of patients during the first 24 h after stroke onset and evidence of early ischaemic lesions was recorded. The stroke cases were divided into subgroups according to the TOAST classification.
Results: The serum levels of hs-CRP of the stroke patients were significantly higher than in the non-stroke patients. The highest percentage of patients with hs-CRP values > 3mg/ L was observed in cases with stroke of undetermined aetiology. Hs-CRP showed a significant diagnostic ability in identifying stroke with an optimal criterion of 4.29 mg/ L. The odds ratios for hs-CRP positive patients indicated an increased risk of stroke and ischaemic lesions. Hs-CRP correlated positively with NIHSS at the time of testing and negatively with stroke evolution.
Conclusion: Hs-CRP is a reliable marker that can be helpful for stroke diagnosis, aetiological classification, clinical manifestation and prognosis. Our results contribute to identifying the characteristics of stroke among the Bulgarian population.
缺血性中风患者的高敏CRP –从危险因素到进化
目的:高敏C反应蛋白(hs-CRP)在脑血管事件的诊断中表现出良好的敏感性。该研究旨在探讨hs-CRP与中风危险因素,神经系统损害,脑缺血和预后之间的关系。
材料和方法:该研究涉及153位患者,分为两类:急性缺血性中风患者和有中风危险因素的患者。收集血液样品以分析hs-CRP的血清水平。采用美国国立卫生研究院卒中量表(NIHSS)测定卒中患者入院和出院时的神经功能障碍。同一组患者在卒中后24小时内进行脑CT检查,并记录早期缺血性病变的证据。将脑卒中患者按年龄分为不同的亚组。
结果:中风患者的血清hs-CRP水平显着高于非中风患者。在病因未明的患者中,hs-CRP值> 3mg / lL的患者百分比最高。 Hs-CRP在识别卒中方面表现出显着的诊断能力,最佳标准为4.29μg/ lL.hs-CRP阳性患者的优势比表明中风和缺血性病变的风险增加。测试时,Hs-CRP与NIHSS呈正相关,与中风演变呈负相关。
结论:Hs-CRP是一种可靠的标志物,有助于中风的诊断,病因分类,临床表现和预后。我们的结果有助于确定保加利亚人口中风的特征。
关键词:高敏C反应蛋白–中风生物标志物–急性缺血性中风
Keywords:
C-reaktivní protein stanovený vysoce senzitivní metodou – biomarkery cévní mozkové příhody – akutní ischemická cévní mozková příhoda
Introduction
Ischaemic stroke is a complex disease in which modifiable and non-modifiable risk factors play an important role [1]. Inflammatory mechanisms are also involved in the stroke pathogenesis [2]. High-sensitive C-reactive protein (hs-CRP) is an acute phase protein that has demonstrated good sensitivity for cardiovascular and cerebrovascular diseases [3,4]. Recent studies have described its positive correlations with the unstable atherosclerotic plaques of the internal carotid artery [5– 7].
In our research we examined the associations between hs-CRP and stroke risk factors and established its connections with neurological impairment, cerebral ischaemia and stroke evolution. We also present data on the characteristics of ischaemic stroke in the Bulgarian population.
Materials and methods
An observational, cross-sectional study was conducted for 6 months (from November 2017 through May 2018) and it enrolled consecutively 153 patients under medical observation in the Clinic of Neurology, UMHAT “St George” – Plovdiv, Bulgaria. It was approved by the Local Ethical Committee. The inclusion criteria included risk factors for ischaemic stroke, such as a history of hypertension, carotid atherosclerosis, diabetes mellitus, atrial fibrillation and cardiovascular disease. The exclusion criteria were autoimmune diseases, cancer and inflammatory and immune diseases. The selection was made by means of medical history, physical examination and laboratory investigations. Two main groups were formed: patients with acute ischaemic stroke and patients with risk factors but without a history of ischaemic stroke.
The information about comorbidity in all patients was collected through medical history, physical examination, laboratory investigations, electrocardiogram monitoring and extracranial carotid duplex ultrasound investigation.
Blood samples from all the patients were collected to analyze the serum level of hs-CRP. In the stroke subgroup, the time from the stroke onset to taking the blood sample was recorded. The serum levels of hs-CRP were determined immunoturbimetrically using an automated clinical-chemical analyzer OLIMPUS AU400 (Beckman Coulter, Brea, CA, USA). Cerebrovascular risk was defined as high as hs-CRP > 3.0 mg/ L, following the reference values given in the Beckman Coulter system manuals and current literature [3,4].
The National Institutes of Health Stroke Scale (NIHSS) was used to determine the neurological disability of the stroke patients. The neurological deficit upon hospital admission and upon discharge was compared in the analysis of stroke evolution.
Cerebral CT was performed on the same group of patients during the first 24 h after the stroke onset. The evidence of early ischaemic lesions in the affected vascular territory was also examined.
A stroke specialist reviewed the data and classified the strokes according to the TOAST classification: large artery atherosclerosis (macroangiopathy), cardioembolic, small vessel disease (lacunar), stroke of other determined aetiology and stroke of undetermined aetiology [8].
The data were analyzed using SPSS (IBM, Armonk, NY, USA), Version 25 [9] and MedCalc Statistical Software (MedCalc, Ostend, Belgium), Version 18.11.3 [10]. The descriptive statistics for continuously measured variables include mean values ± SD; and the frequencies and percentages for nominal and dichotomous variables. The continuously measured variables were screened for violations of normality using the Kolmogorov-Smirnov test. To examine significant differences between the stroke and non-stroke patient groups, we performed independent sample t-tests, when normality was observed (Kolmogorov-Smirnov P > 0.05) and we used the Mann-Whitney U Test when normality was not in place (Kolmogorov-Smirnov P < 0.05). Comparisons of nominal and dichotomous data were performed through cross tabulation, the Chi-square test and Fisher’s exact test. To explore the diagnostic ability of hs-CRP to correctly classify patients as stroke or non-stroke, we applied receiver operating characteristic (ROC) curve analysis and Youden’s index to identify the hs-CRP optimal criterion with the corresponding sensitivity and specificity. The optimal criterion was used to recode hs-CRP into positive and negative cases and calculate the odds ratio (OR) with the incidence of stroke and ischaemic lesions evident on cerebral CT. For the stroke patients, we examined the connection between hs-CRP and the neurological deficit measured by NIHSS, the time of hs-CRP testing, and stroke evolution through correlation analysis. Person-r correlation was employed when both variables were measured continuously and normally distributed, and Spearman-rho correlation in all other cases. The results were interpreted as statistically significant if the P values were < 0.05.
Results
Demographic and clinical characteristics of the patients
The study involved 153 patients of a mean age of 65.08 ± 10.65 years, of whom 82 were males (53.6%). Seventy-nine patients had suffered an ischaemic stroke and 74 had not had an ischaemic stroke. The two groups did not differ significantly in mean age (P = 0.386), gender distribution (P = 0.235), cases with carotid plaques (P = 0.439), cases with hypertension (P = 0.182) or cases with diabetes (P = 0.083). A significantly higher percentage of the patients with ischaemic stroke had nonvalvular atrial fibrillation (P < 0.001) and cardiovascular diseases (P = 0.035) (ischaemic heart disease, valvular heart disease, heart failure and others). The demographic and clinical data are summarized in Tab. 1.
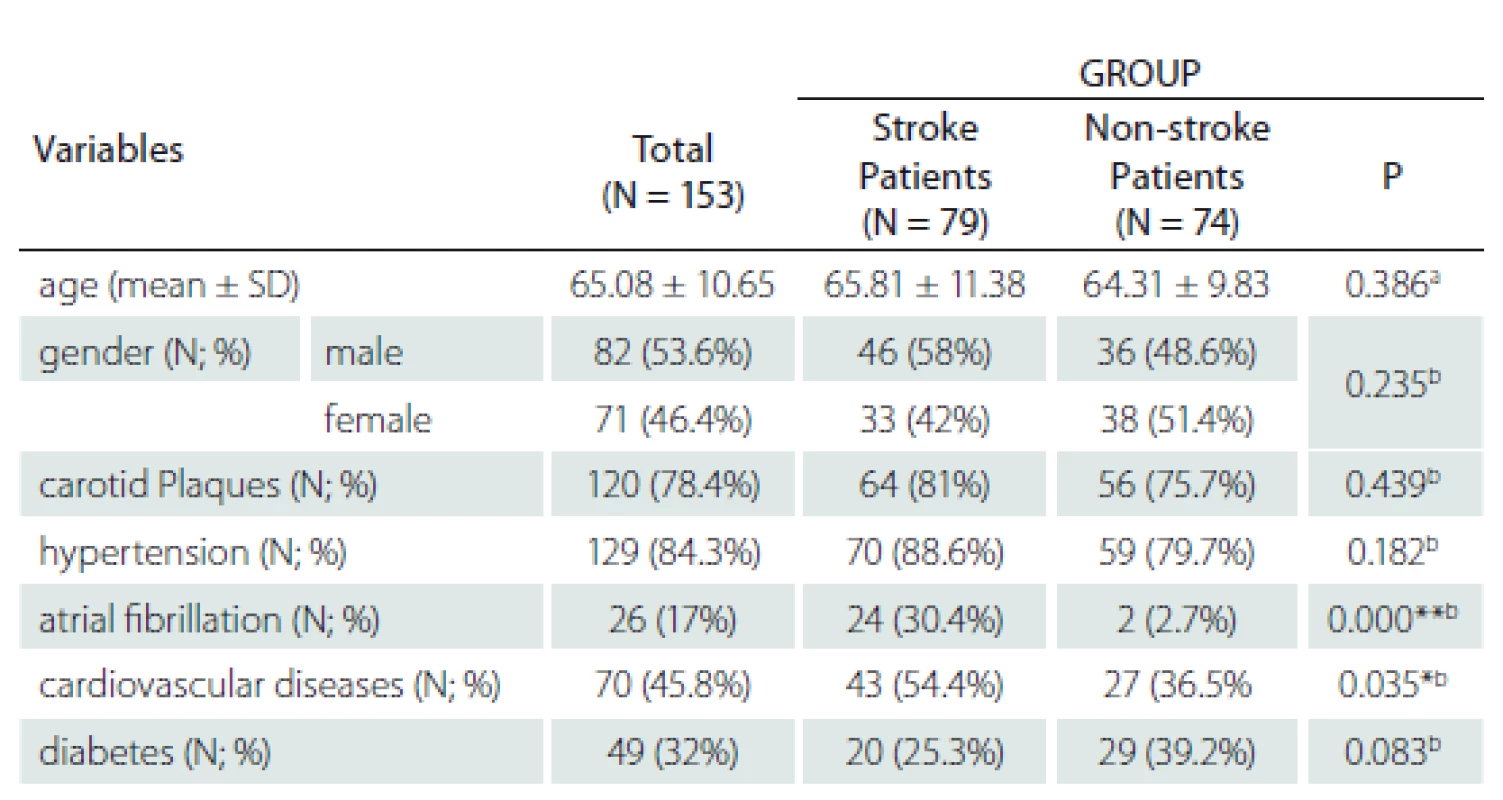
N – number; SD – standard deviation
Diagnostic ability of hs-CRP in ischaemic stroke
A significantly higher hs-CRP level (22.97 mg/ L ± 48.59) was observed in ischaemic stroke patients as compared to non-stroke patients (14.20 mg/ L ± 36.76). The percentage of patients with hs-CRP levels > 3 mg/ L was significantly higher in the stroke patients (71%) vs. 38% in the non-stroke patients; P < 0.001 (Tab. 2).
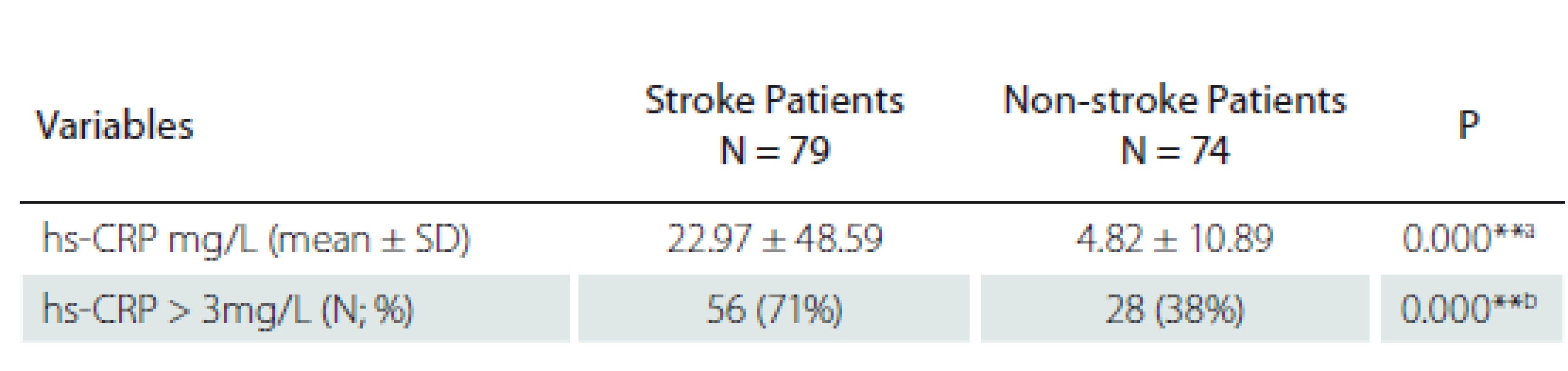
hs-CRP – high sensitivity C-reactive protein; N – number; SD – standard deviation
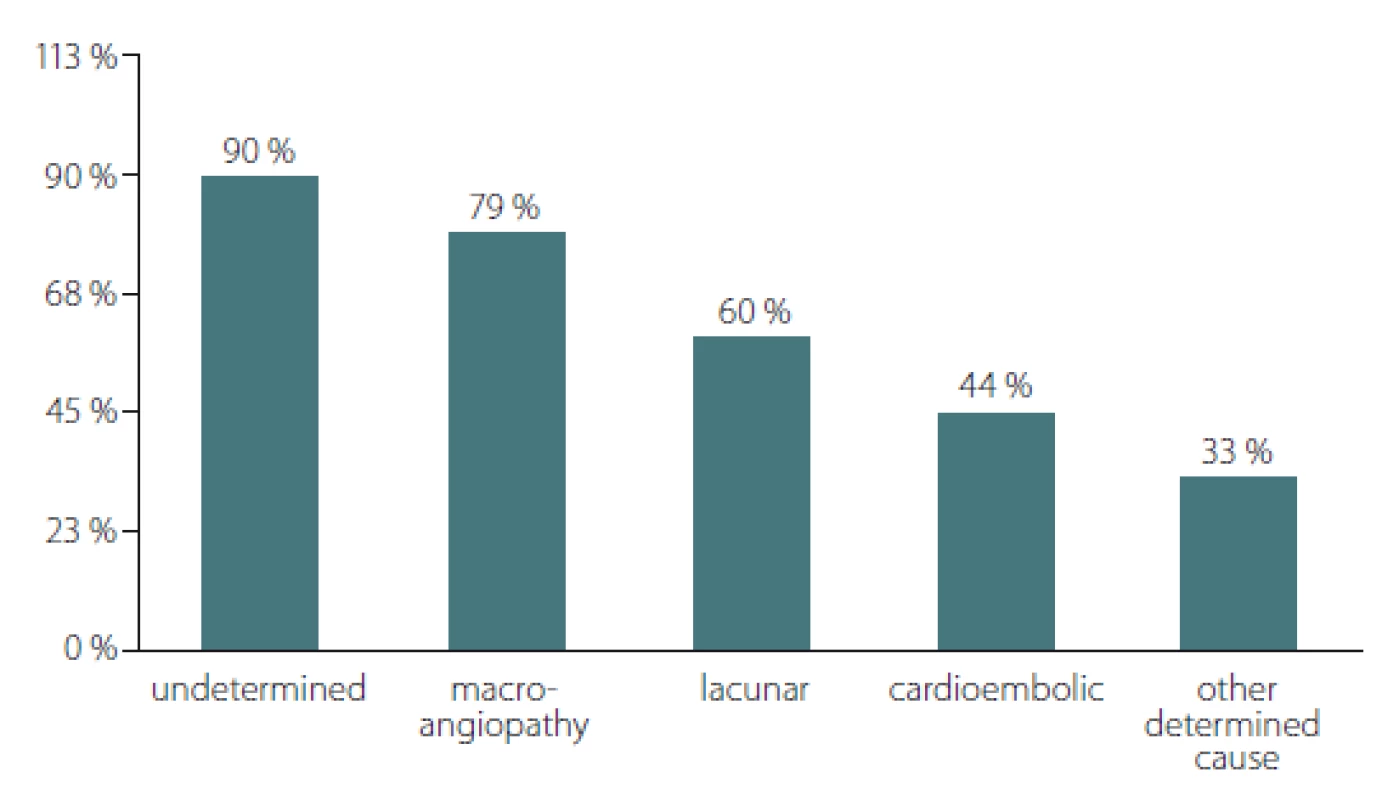
The distribution of hs-CRP values > 3mg/ L across the different types of ischaemic stroke showed significant differences according to the Chi-square test; P = 0.011 (Fig. 1). From the highest to the lowest, the percentage of patients with hs-CRP values > 3mg/ L were as follows: 90% of the patients with stroke of undetermined aetiology (19/ 21); 79.35% of the patients with stroke caused by large artery atherosclerosis (23/ 25); 60% of those with lacunar stroke (9/ 15); 44.4% with cardioembolic stroke (4/ 5); and 33.3% of the patients with stroke of other determined aetiology (2/ 6).
Obr. 1. Distribuce pacientů s hladinami hs-CRP > 3 mg/l napříč typy cévní mozkové
příhody.
* p = 0,011; p-hodnota získána pomocí chí-kvadrát testu
hs-CRP – C-reaktivní protein stanovený vysoce senzitivní metodou
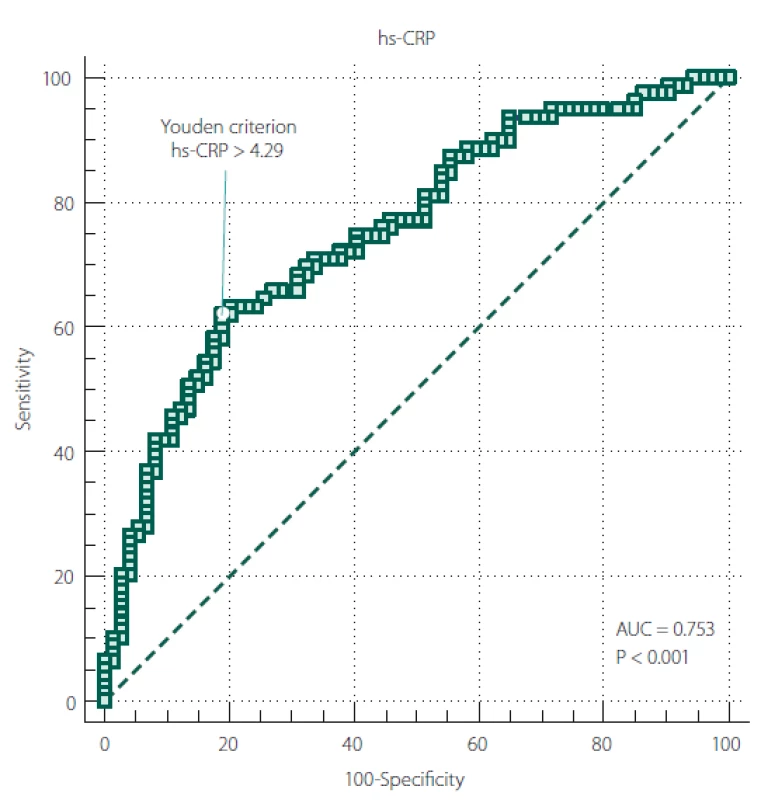
The ROC curve results revealed a significant diagnostic ability of hs-CRP in differentiating stroke from non-stroke patients (area under curve [AUC] = 0.753; P < 0.001) (Tab. 3).
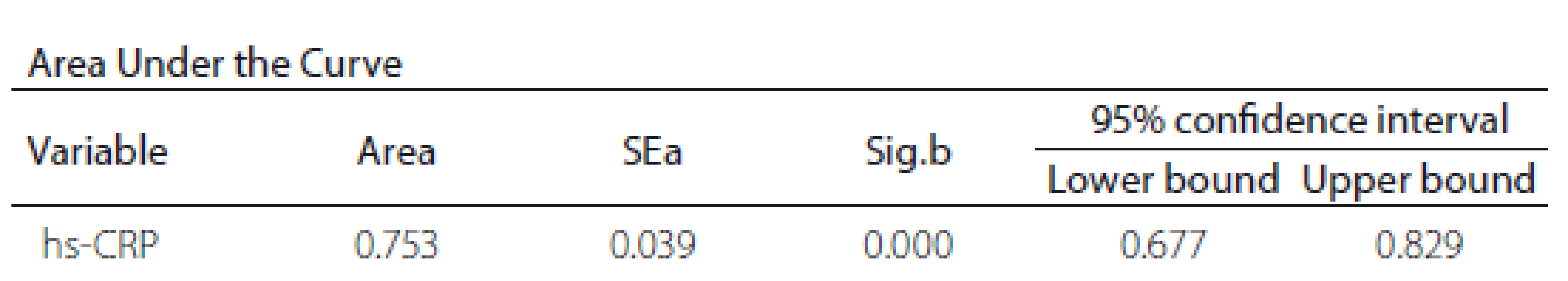
Figure 2 shows the ROC curve between hs-CRP and ischaemic stroke. The universally accepted criterion of hs-CRP > 3mg/ L, associated with increased cardiovascular risk, showed a sensitivity of 70.89 and a specificity of 63.51 in our sample. On the other hand, according to Youden’s index, our data showed hs-CRP > 4.29 mg/ L as the optimal criterion with a sensitivity of 62.03 (95% CI 50.4– 72.7) and a specificity of 81.08 (95% CI 70.3– 89.3).
Obr. 2. ROC křivka mezi hs-CRP a ischemickou CMP.
AUC – plocha pod křivkou; hs-CRP – C-reaktivní protein stanovený vysoce senzitivní
metodou
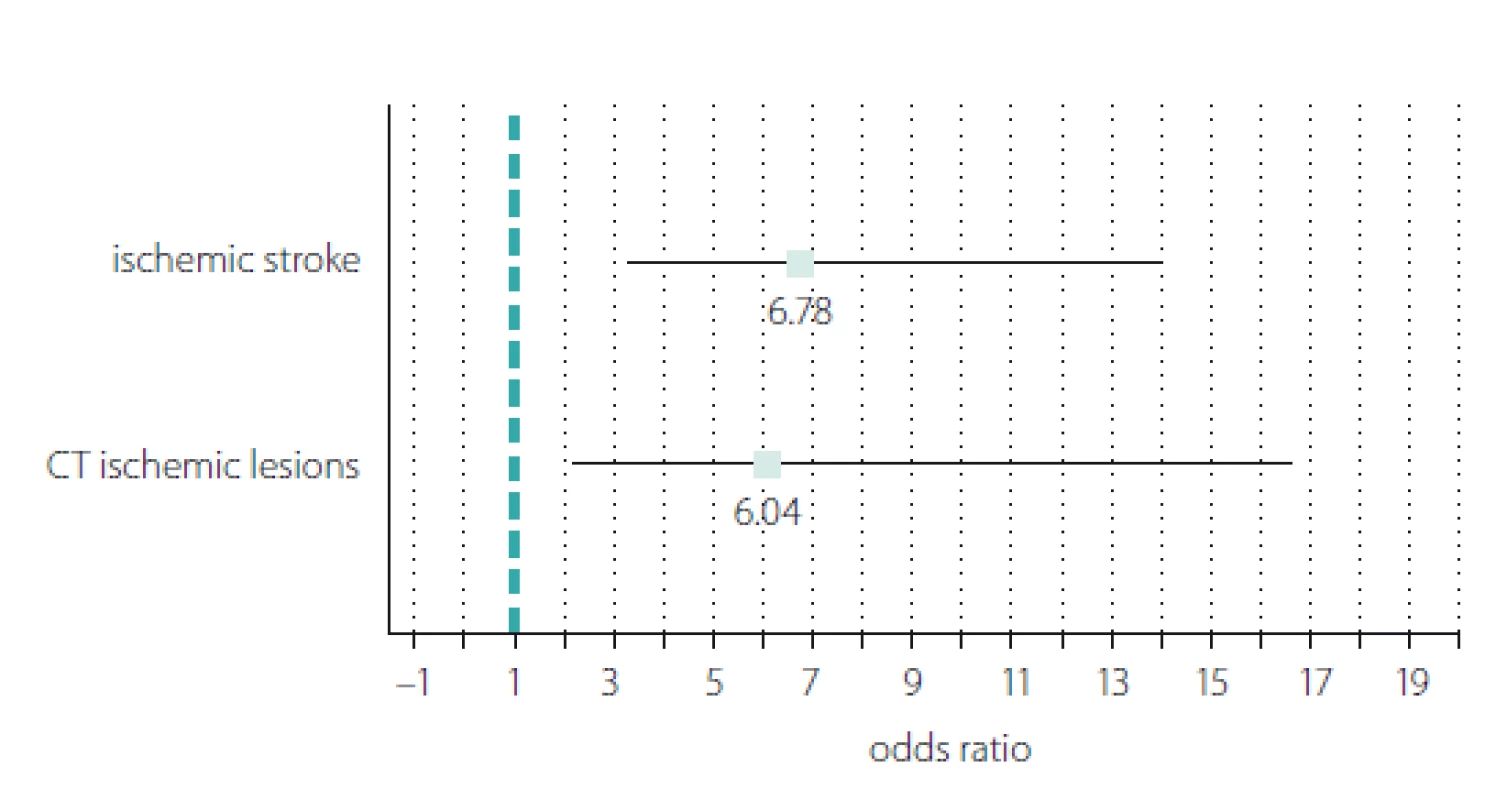
We recoded the hs-CRP variable from a continuous into a dichotomous scale (1 = hs-CRP positive; 0 = hs-CRP negative) using the hs-CRP optimal cut-off value of 4.29 mg/ L and calculated the ORs for the incidence of stroke and early ischaemic lesions observed on cerebral CT (Fig. 3). The results showed significant ORs in both cases. The OR for stroke incidence showed a 6.78 times increased risk of stroke associated with hs--CRP levels above 4.29 mg/ L (OR = 6.78; 95% CI 3.27– 14.04; P < 0.001). The probability of early ischaemic changes seen on CT was 6.04 times higher in stroke patients with hs-CRP > 4.29 mg/ L (OR = 6.04; 95% CI 2.19– 16.62; P < 0.001).
Obr. 3. Znázornění typu „forest plot“ poměru šancí pozitivních případů C-reaktivního proteinu
stanoveného vysoce senzitivní metodou vs. incidence ischemické CMP a ischemických
lézí časně zřetelných na CT.
Correlations between hs-CRP levels and other parameters
For the stroke patients, we examined the connection between hs-CRP and the neurological impairment measured by the NIHSS, the time of hs-CRP testing measured in hours after the stroke, and the stroke evolution. The results are summarized in Tab. 4 and illustrated in Fig. 4.
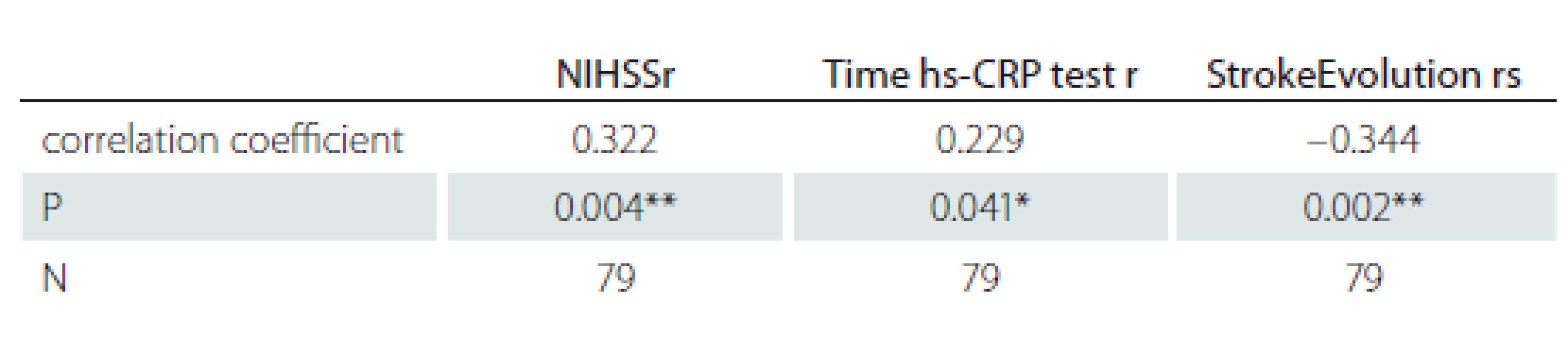
Obr. 4. Vztah hs-CRP a NIHSS, dobou stanovení hs-CRP a vývojem CMP.
Hs-CRP – C-reaktivní protein stanovený vysoce senzitivní metodou; NIHSS – National Institute of Health Stroke Scale
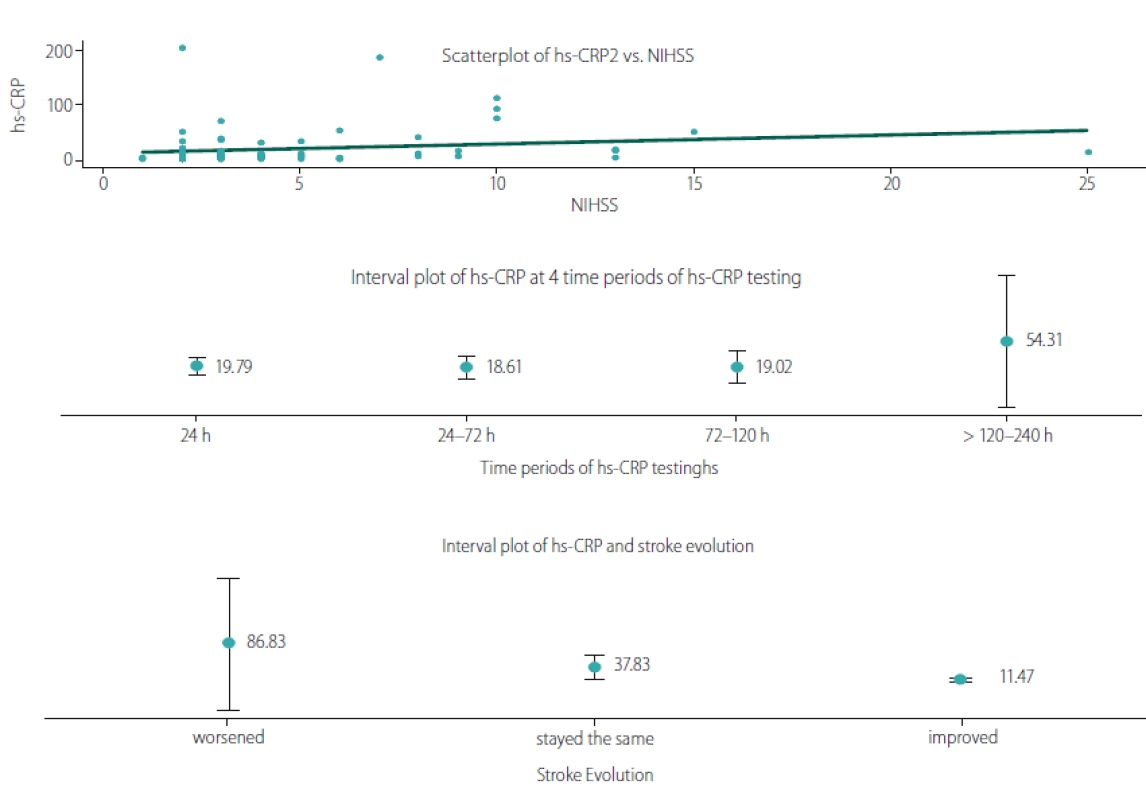
We observed a significant positive linear relationship between hs-CRP and the neurological deficit, measured with NIHSS upon hospital admission (r = 0.322; P = 0.004). The time of the hs-CRP test also correlated positively with the hs-CRP levels (r =0.229; P = 0.041). To illustrate the relationship, we plotted the mean hs-CRP values for four time periods: 24 h > 24– 72 h > 72– 120 h and > 120– 240 h, revealing the period of >120– 240 h as the peak of hs-CRP levels (53.31 mg/ L). When comparing the neurological deficit upon hospital admission and upon discharge we found a negative correlation between hs-CRP and stroke evolution (rs = – 0.344; P = 0.002). Lower hs--CRP levels were associated with those patients whose condition had improved, whereas higher hs-CRP levels were observed in patients whose condition had worsened.
Discussion
The diagnosis of ischaemic stroke is based mainly on clinical evaluation and neuro-imaging techniques [11,12]. The identification of effective biomarkers for stroke diagnosis and prognosis will help in developing proper biological procedures and treatment options [13,14].
Some studies, including the Framingham study, suggest that elevated plasma hs-CRP levels significantly predict the risk of future ischaemic stroke and transient ischaemic attack in the elderly [15,16]. Other studies have identified hs-CRP in patients with ischaemic stroke as a prognostic factor [17,18]. In order to investigate the importance of hs-CRP as a predictor or a short-term prognostic factor, we examined its connection with the stroke risk factors, neurological deficit, cerebral infarcts and evolution.
We analyzed the risk factors in two selected groups – patients with acute ischaemic stroke and patients with risk factors but not suffering from ischaemic stroke. There were no significant differences according to the non-modifiable risk factors such as, gender and age. Comparing the comorbidity in both groups, we found that stroke patients are more affected by nonvalvular atrial fibrillation and cardiovascular diseases. This result may indicate a trend for the Bulgarian population regarding modifiable risk factors and it deserves further investigation.
The study also revealed that Bulgarian stroke patients have significantly higher serum levels of hs-CRP compared to the non-stroke patients. The percentage of patients with hs-CRP levels > 3 mg/ L was significantly higher in the stroke patients compared to the non-stroke patients. Studies of other populations have shown the same prevalence. Indian patients with acute ischaemic stroke had high hs-CRP (> 3 mg/ L) levels [19]. Di Napoli et al found hs-CRP levels (> 0.5 mg/ dL) in 95 Italian patients (74.2%) with acute ischaemic stroke upon admission [20]. Muir et al have detected elevated Hs-CRP (> 10 mg/ L) levels in English stroke patients in the acute phase [21].
Based on the TOAST aetiology classification system, cases of ischaemic stroke are divided into five categories (Fig. 1): stroke after large-artery atherosclerosis (LAA); stroke after cardiac embolism (CE); stroke after small-artery occlusion (SAA); stroke of other determined aetiology (SOE); and stroke of undetermined aetiology (SUE) [8]. We found hs-CRP > 3 mg/ L in all stroke subtypes with maximum prevalence in the SUE (90%), followed by LAA (79.35%) and SAA (60%). Our results correspond with those of Chaudhuri et al [19], who found high hs--CRP levels in 72% of stroke patients with LAA. They are also similar to those of Huang et al [22], who established high hs-CRP levels in 63.9% of stroke patients with large artery atherosclerosis. On the other hand, our findings differ from the results of Den Hertog et al [23] and Dewan et al [24], who reported a lower percentage of stroke patients with LAA and high levels of hs-CRP, 15% and 14.9%, resp. The increased level of hs-CRP observed in this stroke subtype is connected with the concept of increased hs-CRP in unstable and vulnerable carotid plaques [7,25].
In our study the highest percentage of patients with hs-CRP values > 3mg/ L was observed in patients with strokes of undetermined aetiology. This data can be explained due to the involvement of concurrent mechanisms and comorbidity in the group of patients with SUE.
The lowest percentage (33.30%) of patients with hs-CRP values > 3mg/ L was found in the subgroup of patients with SOE. This result is similar to the percentage (40%) reported by Chaudhuri et al [19] and comparable to that of Rajeshwar et al [26].
The ROC curve analysis and Youden’s index provide confirmatory evidence about hs-CRP diagnostic ability in differentiating stroke from non-stroke patients. In our study with Bulgarian patients, we established an optimal criterion of 4.29 mg/ L.
We also examined some clinical aspects of our patients with ischaemic stroke. Analyzing the data about hs-CRP and the ischaemic lesions observed on cerebral CT, we found that patients with high levels of hs-CRP were associated with earlier evidence of ischaemic changes in the affected vascular territory. The ORs for hs-CRP positive patients indicated an increased risk of stroke and early ischaemic cerebral lesions for patients with hs-CRP levels > 4.29 mg/ L. This finding corresponds with previous analyses of patients with ischaemic and haemorrhagic stroke, which have shown that ischaemic lesions are associated with higher serum values of hs-CRP [27,28].
In our study, hs-CRP correlated positively with the neurological assessment made by NIHSS, positively with the time of hs-CRP testing and negatively with stroke evolution. These findings support other investigations which have observed that the hs-CRP level can predict the severity of ischaemic stroke, the ocurrence of a new cerebrovascular accident and an unfavourable stroke outcome [29,30]. Indicula et al found that increased levels of hs-CRP were associated with stroke severity and mortality rate [31]. The authors suggest that measuring hs-CRP upon entry and upon discharge and 1– 3 months later may be useful, as patients with persistently elevated levels of hs-CRP are at a higher risk of future events.
The time of blood testing and its effect on hs-CRP levels has also been studied. Yeh et al examined the hs-CRP levels during the acute and convalescent phases of 160 stroke patients [32]. They found that hs-CRP levels during both phases were significantly and independently predictive of 90-day major adverse neurological events, defined as recurrent stroke, neurological deficit with NIHSS ≥ 8, or death. In our study we revealed the period of > 120– 240 h as the peak of hs--CRP levels (53.31 mg/ L) but the correlation with stroke evolution measuring neurological deficit at admission and discharge was negative. Lower hs-CRP levels were associated with those patients whose condition had improved. This result determinеs the role of hs-CRP as a short-term prognostic factor, regarding its connection with the presence of ischaemic zone and with stroke severity and early outcome.
Conclusion
High-sensitive CRP plays an important role in the definition of stroke risk factors and aetiology, proper diagnosis, clinical manifestation and prognosis. Our results contribute to identifying the characteristics of stroke among the Bulgarian population.
The authors declare they have no potential of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Marieta Peycheva, MD
Department of Neurology
Medical University Plovdiv
66 Peshtersko shosse bul.
4002 Plovdiv
Bulgaria
e-mail: mpeitcheva@yahoo.com
Accepted for review: 25. 5. 2019
Accepted for print: 21. 8. 2019
Sources
1. Boehme AK, Esenwa CE, Elkind M. Stroke risk factors, genetics, and prevention. Circ Res 2017; 120(3): 472– 495. doi: 10.1161/ CIRCRESAHA.116.308398.
2. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics 2016; 13(4): 661– 670. doi: 10.1007/ s13311-016-0483-x.
3. Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97(20): 2007– 2011. doi: 10.1161/ 01.cir.97.20.2007.
4. Wakugawa Y, Kiyohara Y, Tanizaki Y et al. C-reactive protein and risk of first-ever ischemic and hemorrhagic stroke in a general Japanese population: the Hisayama Study. Stroke 2006; 37(1): 27– 32. doi: 10.1161/ 01.STR.0000194958.88216.87.
5. Ammirati E, Moroni F, Norata G. Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm 2015; 2015: 718329. doi: 10.1155/ 2015/ 718329.
6. Puz P, Lasek-Bal A, Ziaja D et al. Inflammatory markers in patients with internal carotid artery stenosis. Arch Med Sci 2013; 9(2): 254– 260. doi: 10.5114/ aoms.2013.34533.
7. Debing E, Peeters E, Demanet C et al. Markers of inflammation in patients with symptomatic and asymptomatic carotid artery stenosis: a case-control study. Vasc Endovascular Surg 2008; 42(2): 122– 127. doi: 10.1177/ 1538574407307406.
8. Adams HP Jr, Bendixen BH, Kappelle LJ et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24(1): 35– 41. doi: 10.1161/ 01.str.24.1.35.
9. IBM SPSS Statistics for Windows, Version 25.0. [online]. Available from URL: https:/ / www.ibm.com/ cz-en/ analytics/ spss-statistics-software.
10. MedCalc Statistical Software version 18.11.3. [online]. Available from URL: https: / / www.medcalc.org.
11. Yu H, Huang Y, Chen X et al. High-sensitivity C-reactive protein in stroke patients – The importance in consideration of influence of multiple factors in the predictability for disease severity and death. J Clin Neurosci 2017; 36: 12– 19. doi: 10.1016/ j.jocn.2016.10.020.
12. Tan JR, Tan KS, Koo YX et al. Blood microRNAs in low or no risk ischemic stroke patients. Int J Mol Sci 2013; 14(1): 2072– 2084. doi: 10.3390/ ijms14012072.
13. Jia L, Hao F, Wang W et al. Circulating miR-145 is associated with plasma high-sensitivity C-reactive protein in acute ischemic stroke patients. Cell Biochem Funct 2015; 33(5): 314– 319. doi: 10.1002/ cbf.3116.
14. Kitagawa K, Hosomi N, Nagai Y et al. Reduction in high-sensitivity C-reactive protein levels in patients with ischemic stroke by statin treatment: Hs-CRP Sub-Study in J-STARS. J Atheroscler Thromb 2017; 24(10): 1039– 1047. doi: 10.5551/ jat.39354.
15. Rost N, Wolf P, Kase C. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham Study. Stroke 2001; 32(11): 2575– 2579. doi: 10.1161/ hs1101.098151.
16. Di Napoli M, Schwaninger M, Cappelli R et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke 2005; 36(6): 1316– 1329. doi: 10.1161/ 01.STR.0000165929.78756.ed.
17. Winbeck K, Poppert H, Etgen T et al. Prognostic relevance of early serial C-Reactive rotein measurements after first ischaemic stroke. Stroke 2002; 33(10): 2459– 2464. doi: 10.1161/ 01.str.0000029828.51413.82.
18. Di Napoli M, Elkind SV, Wagner AP. Role of C-reactive protein in cerebrovascular stroke. Expert Rev Cardiovasc Ther 2011; 9(12): 1565– 1584. doi: 10.1586/ erc.11.159.
19. Chaudhuri JR, Mridula KR, Umamahesh M et al. High sensitivity C-reactive protein levels in acute ischemic stroke and subtypes: a study from a tertiary care center. Iran J Neurol 2013; 12(3): 92– 97.
20. Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke 2001; 32(4): 917– 924. doi: 10.1161/ 01.str.32.4.917.
21. Muir KW, Weir CJ, Alwan W et al. C-reactive protein and outcome after ischemic stroke. Stroke 1999; 30(5): 981– 985. doi: 10.1161/ 01.str.30.5.981.
22. Huang Y, Jing J, Zhao XQ et al. High-sensitivity C-reactive protein is a strong risk factor for death after acute ischemic stroke among Chinese. CNS Neurosci Ther 2012; 18(3): 261– 266. doi: 10.1111/ j.1755-5949.2012.00296.x.
23. den Hertog HM, van Rossum JA, van der WorpHB et al. C-reactive protein in the very early phase of acute ischemic stroke: association with poor outcome and death. J Neurol 2009; 256(12): 2003– 2008. doi: 10.1007/ s00415-009-5228-x.
24. Dewan KR, Rana PV. C-reactive protein and early mortality in acute ischemic stroke. Kathmandu Univ Med J (KUMJ) 2011; 9(36): 252– 255.
25. Garcia BA, Ruiz C, Chacon P et al. High-sensitivity C-reactive protein in high-grade carotid stenosis: risk marker for unstable carotid plaque. J Vasc Surg 2003; 38(5): 1018– 1024. doi: 10.1016/ S0741.
26. Rajeshwar K, Kaul S, Al-Hazzani A et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: correlation with clinical outcome. Inflammation 2012; 35(3): 978– 984. doi: 10.1007/ s10753-011-9401-x.
27. Lal R, Gupta A, Virmani SK et al. High sensitivity C-reactive protein as a prognostic marker in ischaemic and haemorrhagic stroke. Int J Adv Med 2016; 3(2): 372– 377. doi: 10.18203/ 2349-3933.ijam20161094.
28. Roudbary SA, Saadat F, Forghanparast K et al. Serum C-reactive protein level as a biomarker for differentiation of ischemic from haemorrhagic stroke. Acta Med Iran 2011; 49(3): 149– 152.
29. Gong X, Zou X, Liu L et al. Prognostic value of inflammatory mediators in 1-year outcome of acute ischemic stroke with middle cerebral artery stenosis. Mediat Inflamm 2013; 2013: 850714. doi: 10.1155/ 2013/ 850714.
30. Luo Y, Wang Z, Li J et al. Serum CRP concentrations and severity of ischemic stroke subtypes. Can J Neurol Sci 2012; 39(1): 69– 73.
31. Idicula TT, Brogger J, Naess H et al. Admission C-reactive protein after acute ischaemic stroke is associated with stroke severity and mortality: the Bergen stroke study. BMC Neurology 2009; 9: 18. doi: 10.1186/ 1471-2377-9-18.
32. Yeh KH, Tsai TH, Chai HT et al. Comparison of acute versus convalescent stage high-sensitivity C-reactive protein level in predicting clinical outcome after acute ischemic stroke and impact of erythropoietin. J Transl Med 2012; 10: 6. doi: 10.1186/ 1479-5876-10-6.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2019 Issue 5
Most read in this issue
- Treatment of insomnia in the context of neuropathic pain
- Compressive neuropathies as an occupational disease
- Changes of paraspinal muscle morphology in patients with chronic non-specific low back pain
- Endoscopic surgery for lumbar disc herniation – the first experience
