Kappa free light chains in multiple sclerosis – diagnostic accuracy and comparison with other markers
Voľné ľahké reťazce kappa pri roztrúsenej skleróze – diagnostická hodnota a porovnanie s ďalšími markermi
Cieľ: Voľné ľahké reťazce kappa (kappa free light chains; k-fLC) v likvore sú perspektívnym novým markerom intratekálnej syntézy imunoglobulínov (Ig) u pacientov s RS, pričom publikované referenčné hodnoty nie sú jednotné. Cieľom našej práce bolo analyzovať diagnostickú hodnotu k-fLC (hladiny v likvore, kvocientu, indexu) a rutinne vyšetrovaných markerov (IgG index, IgGReiber) v diagnostike RS, v predikcii pozitivity oligoklonálnej skladby (oligoclonal bands; OCB) a u OCB negatívnych pacientov. Metódy: Párované vzorky séra a likvoru boli analyzované v súbore 46 pacientov s RS a 63 kontrolných jedincov s nezápalovými a inými zápalovými ochoreniami CNS. Koncentrácia k-fLC bola meraná imunoturbidimetricky (SPA PLUS®, Freelite®). Pre jednotlivé markery a ich cut-off hodnoty, určené s použitím Youdenovho indexu, boli porovnané ROC krivky, diagnostické senzitivity a špecificity. Výsledky: Optimálne cut-off hodnoty boli: 0,76 pre IgG index; 0,89 mg/l pre IgGReiber; 1,08 mg/l pre hladinu k-fLC v likvore; 0,0994 pre k-fLC kvocient; 18,15 pre k-fLC index. K odlíšeniu RS od control boli zistené najvyššie kombinované diagnostické senzitivity/špecificity pre k-fLC index (0,76/0,98), nasledované k-fLC kvocientom (0,76/0,97), hladinou k-fLC v likvore (0,76/0,95), intratekálnou syntézou IgGReiber (0,70/0,91) a IgG indexom (0,65/0,89). Senzitivita a špecificita OCB vyšetrenia bola 0,83 a 1,00. V predikcii OCB pozitivity malo použitie nových k-fLC markerov excelentnú diagnostickú využiteľnosť so senzitivitou/špecificitou: 0,92/0,99 pre k-fLC index; 0,92/0,97 pre k-fLC kvocient; 0,92/0,96 pre hladinu k-fLC v likvore, v porovnaní s 0,76/0,89 pre IgG index a 0,76/0,86 pre IgGReiber. U OCB negatívnych RS pacientov s použitím navrhnutých cut-off bola najvyššia diagnostická senzitivita (0,38) detekovaná pre IgGReiber, nasledovaná senzitivitou 0,13 pre IgG index a prekvapivo 0,00 senzitiviou pre k-fLC markery. Záver: V diagnostike RS a pri predikcii OCB pozitivity sme detekovali pre všetky analyzované k-fLC markery lepšie diagnostické senzitivity a špecificity v porovnaní s rutinne používaným IgG indexom a IgGReiber, tieto však nedosiahli senzitivitu a špecificitu OCB vyšetrenia. Pre dôkaz intratekálnej syntézy Ig u pacientov so suspektnou RS odporúčame paralelné vyšetrenie OCB spolu s použitím k-fLC indexu a kvocientu, a to predovšetkým ak OCB nález je nejednoznačný alebo negatívny.
Klíčová slova:
roztrúsená skleróza – intratekálna syntéza – voľné ľahké reťazce kappa – index – cerebrospinálny mok – oligoklonálna skladba
Authors:
D. Čierny 1; E. Kantorová 2; M. Škereňová 1,3; E. Kurča 2; J. Lehotský 4; D. Dobrota 1
Authors place of work:
Department of Clinical Biochemistry, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava and, University Hospital Martin, Slovak, Republic
1; Clinic of Neurology, Jessenius Faculty of, Medicine in Martin, Comenius University, in Bratislava and University Hospital, Martin, Slovak Republic
2; Department of Molecular Medicine, Biomedical Center Martin, Jessenius Faculty, of Medicine in Martin, Comenius, University in Bratislava, Slovak Republic
3; Department of Medical Biochemistry, and BioMed, Jessenius Faculty of Medicine, in Martin, Comenius University in, Bratislava, Slovak Republic
4
Published in the journal:
Cesk Slov Neurol N 2021; 84/117(4): 353-360
Category:
Původní práce
doi:
https://doi.org/10.48095/cccsnn2021353
Summary
synthesis in MS patients, with reported discrepant reference values. We aimed to analyse the diagnostic accuracy of k-fLC (CSF concentration, quotient, index) and routinely used markers (IgG index, IgGReiber) in MS diagnostics, in prediction of oligoclonal bands (OCB) positivity and in OCB negative patients. Methods: Serum/CSF paired samples were analysed from 46 MS patients and 63 controls with non-inflammatory and non-MS inflammatory diseases of CNS. The concentrations of k-fLC were measured immunoturbidimetrically (SPA PLUS®, Freelite®). The ROC curves, diagnostic sensitivities and specificities were analysed with respect to the cut-offs, which were determined using Youden’s index. Results: The optimal cut-off values were defined as: 0.76 for IgG index; 0.89 mg/L for IgGReiber; 1.08 mg/L for CSF k-fLC level; 0.0994 for k-fLC quotient; 18.15 for k-fLC index. To distinguish MS patients from controls, the highest combined sensitivities/specificities were observed for k-fLC index (0.76/0.98), followed by k-fLC quotient (0.76/0.97), CSF k-fLC level (0.76/0.95), IgGReiber (0.70/0.91) and IgG index (0.65/0.89). The OCB detection showed the sensitivity 0.83 and the specificity 1.00. In prediction of OCB positivity, the novel k-fLC markers showed excellent diagnostic accuracy with sensitivities/specificities: 0.92/0.99 for k-fLC index, 0.92/0.97 for k-fLC quotient, and 0.92/0.96 for CSF k-fLC level, compared to 0.76/0.89 for IgG index and 0.76/0.86 for IgGReiber. Applying our cut-offs in OCB negative MS patients, the highest diagnostic sensitivity (0.38) was found for IgGReiber, followed by 0.13 for IgG index, and surprisingly by 0.00 for all k-fLC markers. Conclusion: In MS diagnostics and in prediction of OCB positivity, all novel k-fLC markers showed better sensitivities and specificities than routinely used IgG index and IgGReiber, but did not reach those of OCBs. To proove the intrathecal Ig synthesis in patients with suspected MS, we recommend to use k-fLC index and quotient along with OCB, especially if OCB findings are equivocal or negative.
Keywords:
Multiple sclerosis – intrathecal synthesis – kappa free light chains – index – cerebrospinal fluid – oligoclonal bands
Introduction
The detection of intrathecal immunoglobulin (Ig) synthesis is an important diagnostic tool in multiple sclerosis (MS), as well as in other inflammatory, neoplastic and autoimmune diseases of the CNS [1–2]. MS is the most common autoimmune demyelinating disease of CNS in young adults, characterised by inflammation, attacks of demyelination and chronic neurodegeneration [3]. MS is diagnosed using the McDonalds criteria, which comprehensively consider patients‘ neurological clinical symptoms, MRI, evoked potentials, and cerebrospinal fluid (CSF) examination [1–3]. The intrathecal synthesis of Ig in MS is a result of pathological immune system activation, which is driven by tertiary lymphatic organs in the CNS through classical and non-specific pathways [4]. The determination of intrathecal Ig synthesis is performed by “gold standard“ qualitative oligoclonal band (OCB) detection with a complementary quantitative IgG analysis, routinely analysed in each patient suspected of having MS [5]. However, the results of these laboratory methods can be inconclusive or unclear in some patients [6], hindering further appropriate therapeutical intervention. In the last decades, immunoglobulin free light chains (fLC) have been widely studied as promising new markers of intrathecal Ig synthesis in MS [7]. Both types of fLC, kappa (k-fLC) and lambda (l-fLC), are side products of Ig synthesis, reflecting recent immunological situation within the CNS of MS patients [8]. Due to a relatively small molecular weight compared to albumin, fLC can easily pass the blood-CSF barrier. Thus, in patients with increased fLC level in serum, determining intrathecal synthesis by fLC absolute level in CSF can result in false positivity [9]. This can be eliminated by using fLC quotient, which also takes into account a serum fLC level, or fLC index, which also considers CSF to serum albumin quotient and thus the possible blood--CSF barrier dysfunction. Therefore, fLC index seems to be the most representative new marker able to provide relevant information about intrathecal synthesis of Ig. In MS, better diagnostic relevance was found for k-fLC than for l-fLC [10–13]. However, several studies that defined k-fLC index cut-off with proper diag- nostic sensitivity and specificity in MS gave inconsistent results with values ranging from 3,61 to 20 [11–17]. The situation is similar regarding the k-fLC quotient cut-off values, either Presslauer’s individual limit k-fLC quotient [18] or the absolute cut-off value [9].
In general, k-fLC markers are not routinely used to determine intrathecal Ig synthesis in Slovakia. Thus, aims of our study were: 1) to assess whether the novel k-fLC markers of intrathecal Ig synthesis (absolute CSF k-fLC level, quotient, index) are useful compared to the routinely used markers (IgG index, local intrathecal IgG synthesis – IgGReiber), and 2) to analyse the diagnostic accuracy of k-fLC marker cut-off values in the diagnostics of MS, in prediction of OCB positivity, and in OCB negative MS patients.
Material and methods
Patients and controls
In our study, k-fLC levels were measured in paired CSF and serum samples of 46 MS patients and 63 control subjects. The patients with clinically isolated syndrome (CIS) and MS as well as controls were hospitalised at the Clinic of Neurology, Jessenius Faculty of Medicine in Martin and University Hospital Martin, Slovak Republic. A clinically definitive MS diag- nosis was established according to the McDonald criteria [1]. The control group consisted of patients with non-inflammatory CNS diseases (N-IND, N = 34) and non-MS inflammatory CNS diseases (IND, N = 29). All control individuals were OCB negative – OCB type 1 was found in 17 patients with N-IND and 14 with IND; OCB type 4 in 17 patients with N-IND and 15 with IND. The N-IND subgroup consisted of patients with Parkinson’s disease (N = 3), Alzheimer’s disease (N = 3), amyotrophic lateral sclerosis (N = 4), hereditary spinocerebellar ataxia (N = 1), hereditary adrenoleukodystrophy (N = 2), spinal stenosis (N = 3), cerebral ischemia (N = 2), tension headache (N = 2), microvascular encephalopathy (N = 2), Charcot Marie Tooth disease (N = 1), epilepsy (N = 3), myasthenia gravis (N = 1), facial nerve palsy (N = 3), other cranial nerve palsy (N = 2) and cortical atrophy (N = 2). The IND subgroup included patients with meningitis, encephalitis and myelitis (N = 13), cranial neuritis (N = 4), polyradiculoneuritis (N = 3), chronic inflammatory demyelinating polyneuropathy (N = 7) and optic neuritis (N = 2).
Clinical data, blood and CSF samples were taken over a period between March 2016 and February 2019 during the patients‘ hospitalisation at the Clinic of Neurology. The MS group consisted of 38 OCB positive patients – 20 with OCB type 2 and 18 with OCB type 3, and 8 OCB negative MS patients – 4 with OCB type 1 and 4 with OCB type 4. Clinical characteristics of MS patients and controls are shown in Tab. 1.
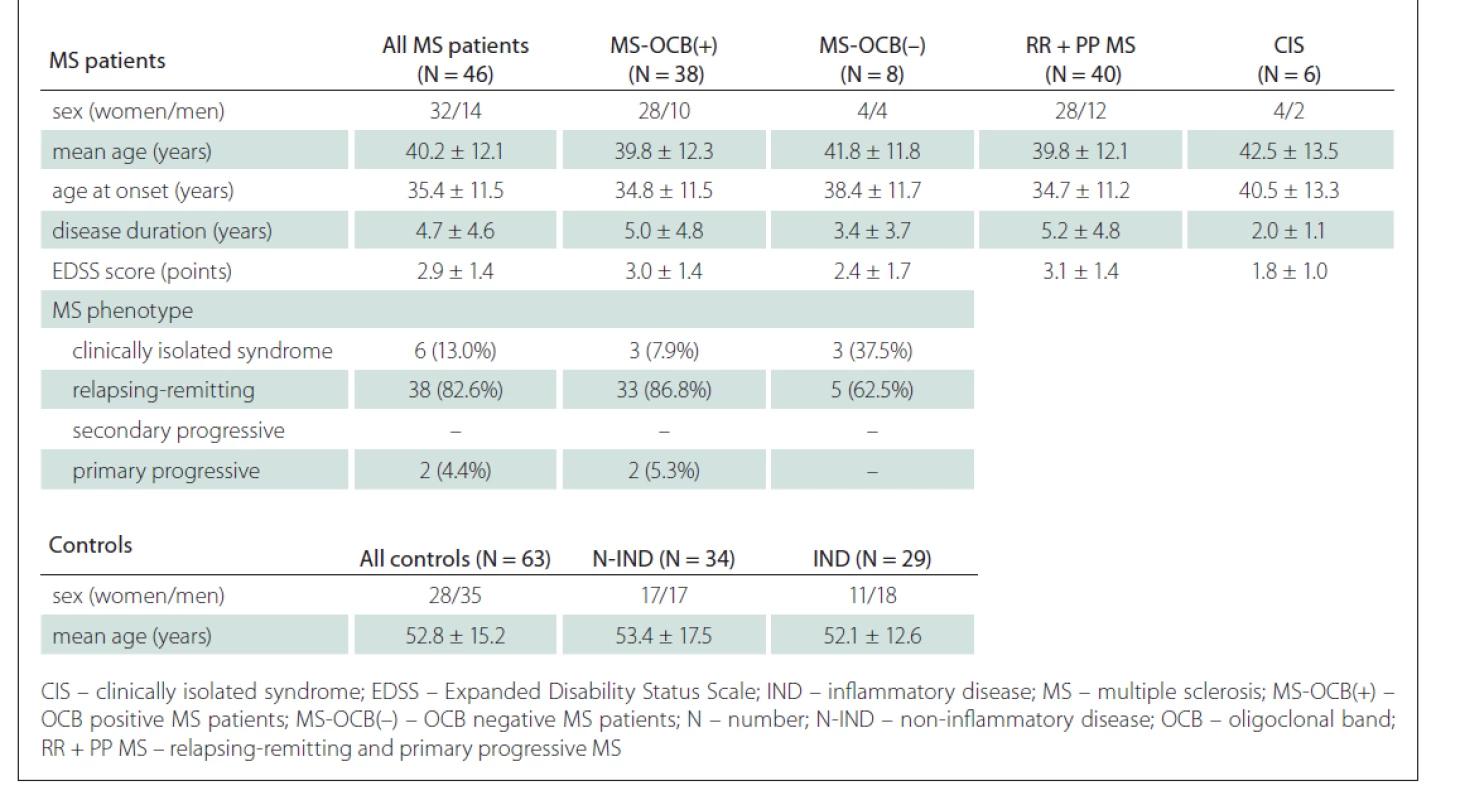
All individuals involved in the study were Slovak. The control group subjects were older (mean age 52.8 ± 15.2 years) than MS patients (mean age 40.2 ± 12.1 years; P < 0.001). The MS patient group consisted of 44.4% women and 55.6% men, the control group consisted of 69.6% women and 30.4% men, and there was a significant difference in sex distribution between the two groups (P = 0.011).
Sample processing and analysis
Peripheral venous blood samples were taken and dispensed into 8.5 mL Vacutainer collection tubes containing gel&clot activator, cat. no. 367953 (Vacutest Kima, Arzergrande, Italy). The samples were centrifuged at 4,000 g at 5°C for 5 min. The CSF samples were taken into 12 mL sterile tubes, centrifuged at 4,000 g at 5°C for 5 min and subsequently the supernatant was analysed. Albumin and total protein concentrations in serum and CSF were measured photometrically in Olympus AU680/AU700 analyser (Beckman Coulter, Brea, CA, USA). The serum albumin method (Albumin kit, cat. no. OSR 6202; Beckman Coulter, Brea, CA, USA) is a modification of the Doumas and Rodkey procedures. The bromocresol green reacts with albumin at pH 4.2 to form an intense-green complex, the absorbance is measured bichromatically (600/800 nm), and the sensitivity is 0.07 g/L. Cerebrospinal fluid albumin was measured using a turbidimetric method (Urine/CSF Albumin kits, cat. no. B38858; Beckman Coulter, Brea, CA, USA). CSF abumin reacts with anti-human serum albumin antibody forming light-scaterring immune complexes, and the change in absorbance is measured at 380 nm (reference wavelength 800 nm) with the sensitivity of 1.3 mg/L. The serum total protein procedure (Total protein kit, cat. no. OSR6232; Beckman-Coulter, Brea, CA, USA) is based on a reaction with cupric ions in an alkaline solution producing a violet-coloured complex (Weichselbaum modification), and the absorbance proportional to the protein concentration is measured at 540/660 nm. The sensitivity of the method is 0.77 g/L. CSF protein reacts with pyrogallol red and molybdate forming a red coloured complex, the absorbance is measured at 600 nm, and the sensitivity is 0.02 g/L (Total protein UC FS kit, cat. no. 102109910026; Diasys, Holzheim, Germany). Immunoglobulin G levels in serum were measured immunoturbidimetrically in an automated analyser Architect ci4100 (Abbott, Chicago, IL, USA) using Immunoglobulin G kits (cat. no. 9D99-21; Abbott, Chicago, IL, USA). The immunoglobulin G level in CSF was measured immunoturbidimetrically in an analyser SPA PLUS® (Binding Site, Birmingham, UK) using Human IgG CSF kits (cat. no. NK004.L.S; Binding Site, Birmingham, UK). The OCB analysis was performed by laboratory technicians using immunofixation on HYDRASIS 2 system (Hydragel 9 CSF Isofocusing kit, cat. no. 4355; Sebia, Lisses, France). The OCBs were subsequently determined by experienced medical staff and reported as positive or negative. For further use, the aliquotes of serum and CSF samples were stored in Eppendorf tubes at –80 °C. The level of k-fLC in serum and CSF was measured by immunoturbidimetry in SPA PLUS® analyser (Binding Site, UK) according to the manufacturer‘s protocol using Freelite® Human Kappa Free kits (cat. no. LK016.S and LK016.L.S; Binding Site, UK). The kits contain specific latex-bound antibodies that react with k-fLC in the sample forming non-soluble immunocomplexes and decreasing the light beam intensity that is proportional to the concentration of k-fLC in the sample.
Statistical analysis
The albumin quotient was calculated as: (CSF albumin concentration) / (serum albumin concentration). The IgG quotient was calculated as: (CSF IgG concentration) / (serum IgG concentration). The IgG index was calculated as: (CSF IgG concentration x serum albumin concentration) / (serum IgG concentration x CSF albumin concentration). Intrathecal local IgG synthesis was calculated as: IgGReiber = (QIgG – Qlim (IgG)) x IgGserum [ mg/L]; IgGith = 100 x (1 – Qlim (IgG) /QIgG) [%]; using Qlim (IgG) = 0,93 x √ (Qalb2 + 6 x 10–6) – 1,7 x 10–3 [2]. The quotient of k-fLC was calculated as: (CSF k-fLC concentration) / (serum k-fLC concentration). The individual k-fLC limit quotient was calculated as Qlim (k-fLC) Presslauer = 0,9358 x Qalb0,6687 [18]. The index of k-fLC was calculated as: k-fLC index = Qk-fLC / Qalb. The statistical analysis was performed using the SVS 7 software (SNP & Variation Suite v7.6.11, Golden Helix, Bozeman, MT, USA). For all analysed markers, with respect to the cut-offs, diagnostic sensitivities and specificities were calculated. The sensitivities over all false-positive rates were displayed in receiver operating characteristic (ROC) curves and, as a measure of discrimination, an area under the curve (AUC) value was obtained using the nonparametric estimation method. The optimal cut-off values were determined using the Youden’s J statistic. To detect whether there are statistically significant differences between the ROC curves (AUC values) of analysed markers, we used the z-statistic method of Delong et al [19]. To test statistical differences in age and sex distribution of the studied groups, we used the Mann-Whitney test and Fisher’s exact test.
Results
In the first part of our study, we asessed whether the novel markers of intrathecal immunoglobulin synthesis (CSF k-fLC level, quotient, index) can be more useful than the routinely used markers (IgG index, IgGReiber) in the diagnostics of MS, in prediction of OCB positivity and in OCB negative MS patients. Nonparametrically estimated AUC values, showing discrimination ability of the markers, are shown in Tab. 2.
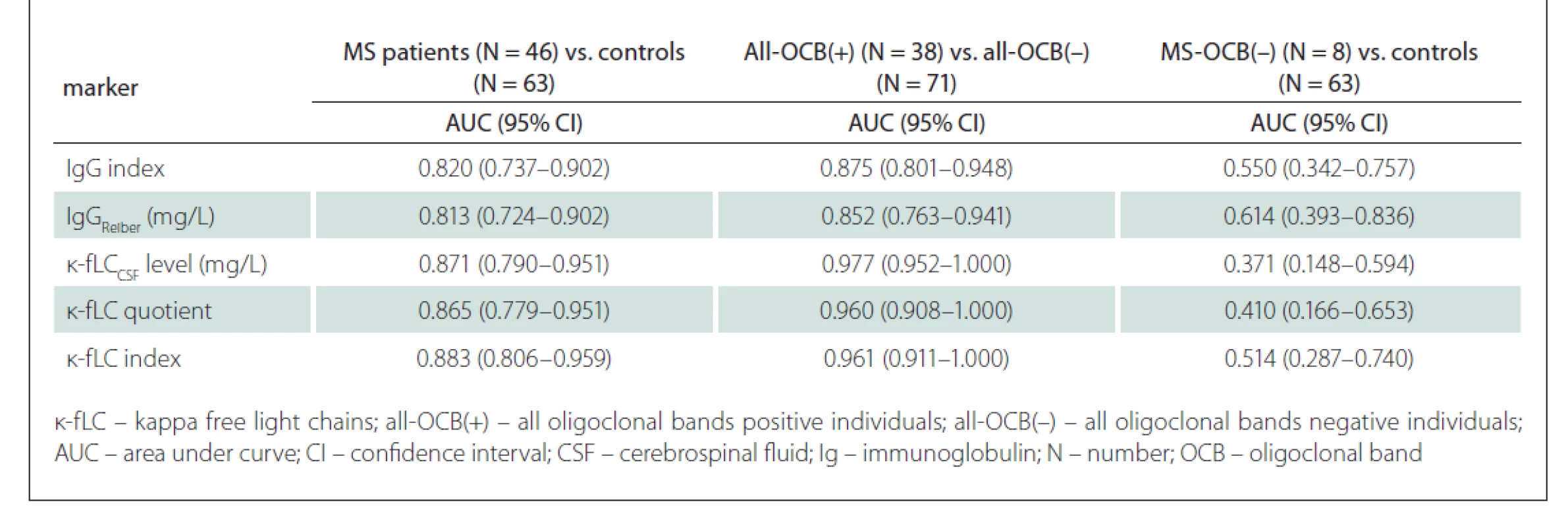
After analysing ROC curves for all MS patients vs. controls, we found no statistically significant differences between AUC values of the novel markers (CSF k-fLC level, k-fLC quotient, k-fLC index) and IgG index (P = 0.226, 0.321, and 0.162 respectively) and IgGReiber (P = 0.185, 0.260, and 0.113 respectively). However, comparing ROC curves of the markers for their ability to predict OCB positivity provided very interesting results. We found significantly higher AUC values when we compared the CSF k-fLC level, k-fLC quotient and k-fLC index with the AUC of IgGReiber (P = 0.0025, 0.026 and 0.023 respectively), and k-fLC CSF level with IgG index (P = 0.008). The AUC values of k-fLC quotient and k-fLC index were also higher than AUC of IgG index, showing a strong trend toward significance (P = 0.065 and 0.059 respectively). However, the ability to discriminate OCB negative MS patients from controls was not proven for any of the analysed markers because the AUC values did not significantly differ from value 0.5, and thus were not further compared (data are shown in Tab. 2). The marker ROC curves for diagnostics of MS are shown in Fig. 1 (including optimal cut-off values). Fig. 2 shows the ROC curves in prediction of OCB positivity.
Optimal cut-off values (Youden´s index, J) were determined as: 0.76 for IgG index (J = 0.541),
0.89 mg/L for IgGReiber (J = 0.600), 1.08 mg/L for κ-fLCCSF level (J = 0.713), 0.0994 for κ-fLC quotient
(J = 0.729) and 18.15 for κ-fLC index (J = 0.745)
κ-fLC – kappa free light chains; CSF – cerebrospinal fluid; CTL – control group; Ig – immunoglobulin;
ROC – receiver operating characteristic
Obr. 1. ROC krivky pre analyzované markery intratekálnej syntézy Ig v diagnostike SM.
Optimálne definované hodnoty cut-off (Youdenov index, J) boli: 0,76 pre IgG index
(J = 0,541); 0,89 mg/l pre IgGReiber (J = 0,600); 1,08 mg/l pre hladinu κ-fLC v likvore
(J = 0,713); 0,0994 pre κ-fLC kvocient (J = 0,729) a 18,15 pre κ-fLC index (J = 0,745).
κ-fLC – voľné ľahké reťazce kappa; CSF – cerebrospinálny mok; CTL – kontrolná skupina;
Ig – imunoglobulín; ROC – receiver operating characteristic

κ-fLC – kappa free light chains; Ig – immunoglobulin; OCB – oligoclonal band;
ROC – receiver operating characteristic
Obr. 2. ROC krivky pre analyzované markery intratekálnej syntézy Ig v predikcii OCB pozitivity.
κ-fLC – voľné ľahké reťazce kappa; Ig – imunoglobulín; OCB – oligoklonálny pás;
ROC – receiver operating characteristic

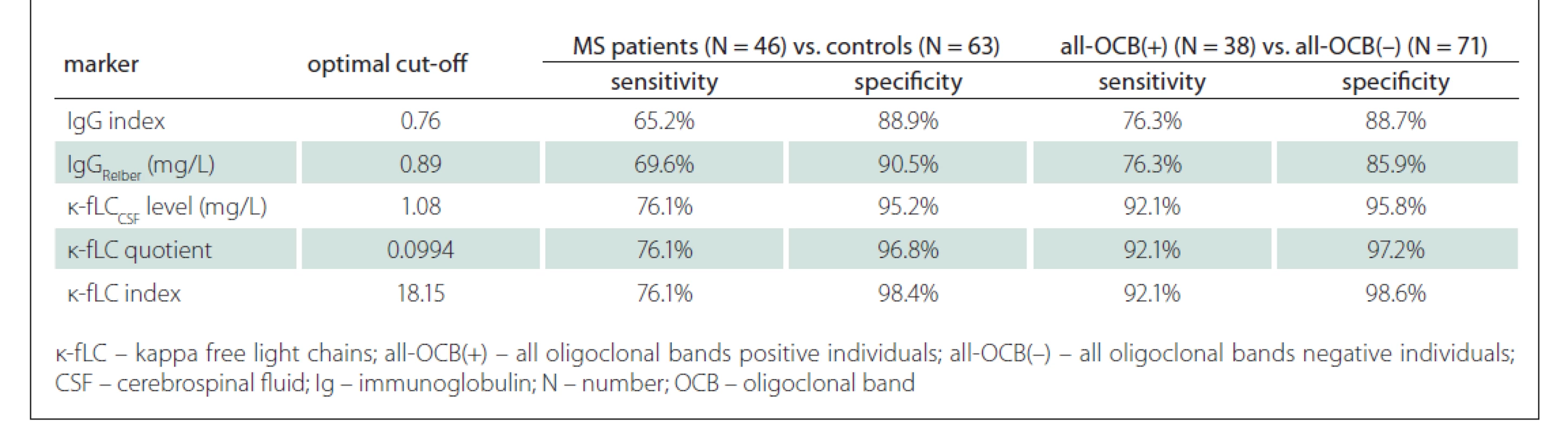
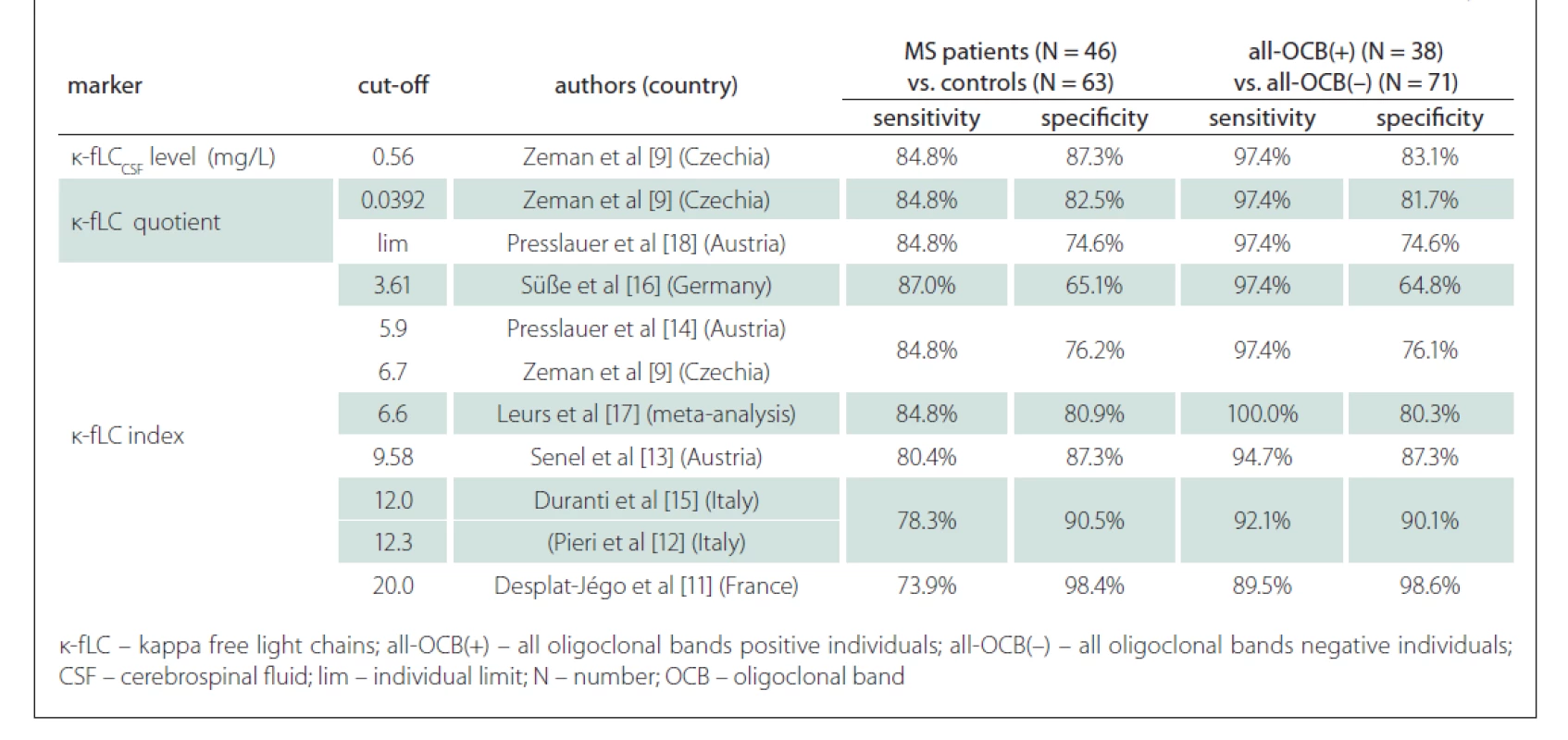
Since the measurement of novel k-fLC markers (CSF k-fLC level, quotient, index) is not routinely used in the diagnostics of intrathecal Ig synthesis and the results of the studies determining the cut-off values were not concordant, we defined our own cut-offs. In our cohort, we analysed the diagnostic characteristics of both our own cut-offs as well as those suggested in the literature and compared them in relation to the diagnostics of MS and prediction of OCB positivity. The results of our analysis, represented by diagnostic sensitivities (number of patients with marker values higher than the cut-off / number of all patients) and diagnostic specificities (number of controls with marker values lower than the cut-off / number of all controls), are shown in Tab. 3. The sensitivity of OCB detection in MS patients was 82.6% (38/46), the specificity in controls was 100% (63/63), since all of them were OCB negative. The diagnostic sensitivities and diagnostic specificities obtained in our cohort using the cut-off values from previous studies are summarised in Tab. 4.
Discussion
Apart from the OCB detection, k-fLC measurement in CSF has been recently evaluated to be the relevant marker of intrathecal Ig synthesis in MS patients [13]. However, most Slovakian laboratories, including ours, do not yet employ the novel k-fLC markers (CSF k-fLC level, k-fLC quotient, and k-fLC index) in routine diagnostic use, and the reference values reported in relevant studies are discrepant [9,11–18]. Due to these facts, we aimed to define the optimal cut-offs of k-fLC markers and evaluate their diagnostic performance in the diagnostics of MS and in the prediction of OCB positivity. To assess the utility of different k-fLC markers, we also compared their diagnostic accuracy with routinely used markers as IgG index and local intrathecal IgGReiber synthesis. In line with the fact, that k-fLC measurement has also been suggested to be a promising marker of intrathecal Ig synthesis in OCB negative MS patients [20], we also aimed to assess the feasibility of using k-fLC markers in this group of MS patients.
In this study, we analysed 46 paired serum/CSF samples from MS patients and 63 paired serum/CSF samples from OCB negative controls with other inflammatory and non-inflammatory diseases of CNS. Since the OCBs are still recommended as a „gold standard“ marker for intrathecal Ig synthesis [2,5], all involved individuals underwent OCB detection during their diagnostic procedure, and it was a criterion for stratifying MS patients into subgroups.
Firstly, in MS patients and controls, we used Youden’s index (J) to define our own optimal cut-off values, which were 0.76 for IgG index (J = 0.541), 0.89 mg/L for IgGReiber (J = 0.600), 1.08 mg/L for CSF k-fLC level (AUC = 0.871), 0.0994 for k-fLC quotient (AUC = 0.865) and 18.15 for k-fLC index (AUC = 0.883). Unexpectedly, when we compared the k-fLC marker AUC values with those of IgG index (AUC = 0.820) and local IgGReiber (AUC = 0.813), the statistical analysis did not show any significant difference. However, when we used these cut-offs to distinguish MS patients from controls, the highest combined sensitivity and specificity was showed for k-fLC index (76.1 and 98.4%), followed by k-fLC quotient (76.1 and 96.8%), CSF k -fLC level (76.1 and 95.2%), IgG Reiber (69.6 and 90.5%) and IgG index (65.2 and 88.9%). These data indicate the excellent utilizability of novel k-fLC markers in MS diagnostics. From those, we would certainly prefer the k-fLC quotient and index, due to the possible false positivity of CSF k-fLC level caused by its physiological dependence on serum k-fLC concentration. Typically, increased serum k-fLC concentrations are observed in patients with multiple myeloma and other monoclonal gammopathies, and this marker is used in diagnostics, monitoring of treatment response and also as a prognostic marker [21]. For easy comparison of the diagnostic performance of our cut-offs with those suggested by other studies, the sensitivities and specificities calculated in our cohort are summarised in Tab. 3 and in Tab. 4.
In a large multicenter study of Leurs et al [17], the k-fLC index cut-off 6.6 was defined in 745 European patients with the 88% sensitivity and 83% specificity in the diagnostics of MS. In our cohort, the k-fLC index 6.6 had similar sensitivity and specificity, 84.8% and 80.9%, respectively. In the study of Presslauer et al [14], the sensitivity and specificity of k-fLC index cut-off 5.9 in MS diagnostics has been 96% and 86%, respectively (AUC = 0.954), which is about 10% higher than those for the index 5.9 in our cohort (84.8 and 76.2%). This could be caused by a relatively higher proportion of OCB negative MS patients in our study. Compared to the results of these studies, the 76.1% sensitivity of our k-fLC index cut-off 18.15 was 10–20% lower, but in clinical practice, this could be balanced by its very high specificity, which reached 98.4%. However, in MS diagnostics, the 76.1% sensitivity and 98.4% specificity of our optimal k-fLC index 18.15 were lower than those of OCB detection (82.6% sensitivity and 100% specificity) in our cohort. This does not correlate with the findings of metaanalysis of Leurs et al [17], which showed k-fLC index 6.6 to be more sensitive than OCB (88 vs. 82%) at the expense of a lower specificity than OCB (83 vs. 92%). This could be explained by a significantly smaller group in our study, as well as by the difference in used k-fLC index cut-offs, since our findings using k-fLC index 6.6 were similar.
Regarding the prediction of OCB positivity, our study found the novel k-fLC markers to be better than the routinely used markers. Interestingly, we found the AUC values of CSF k-fLC level (0.977), k-fLC quotient (0.960) and k-fLC index (0.961) to be significantly higher compared to 0.875 of IgGReiber (P = 0.0025, 0.026 and 0.023, respectively) and also higher when compared to 0.852 of IgG index (P = 0.008, 0.065 and 0.059, respectively). Furthermore, when we used the suggested cut-offs (1.08 mg/L for CSF k-fLC level, 0.0994 for k-fLC quotient, 18.15 for k-fLC index) in all OCB positive and all OCB negative individuals, all these markers showed 15.8% higher sensitivity and about 10% higher specificities when compared to IgG index cut-off 0.76 and IgGReiber cut-off 0.89 mg/L (see Tab. 3). When determining OCB positivity, out of all markers investigated, the highest and practically appliable combined sensitivity and specificity was found for k-fLC index cut-off 18.15, which showed excellent 92.1% sensitivity and 98.6% specificity, followed by k-fLC quotient 0.0994 showing 92.1% sensitivity and 97.2% specificity, and CSF k-fLC level with 92.1% sensitivity and 95.8% specificity. Considering all these data, we suggest to use k-fLC index or quotient also in the prediction of OCB positivity, as it is more accurate than simple k- CSF fLC level measurement, especially in patients with impaired blood-CSF barrier or higher k-fLC level in serum. From all the suggested k-fLC index cut-offs from other studies that were applied in our cohort (reviewed in Tab. 4), the closest correlation with high sensitivity and specificity (92.1 and 90.1%) was reached using the cut-off 12.0, that was defined by Duranti et al. [15]. In addition to that, in their group of 80 Italian patients, this cut-off detected MS with the 95% sensitivity and 91% specificity (AUC = 0.947).
In our study, we also assessed the diagnostic usefullness of the novel markers in OCB negative MS patients, which was surprisingly lower than that of the routine markers. Applying our cut-offs in this problematic group of MS patients, out of all markers investigated, the highest diagnostic sensitivity (37.5%) was found for local IgG synthesis, followed by the 12.5% sensitivity of IgG index, and surprisingly the 0% sensitivity of CSF k-fLC level 1.08 mg/L, k-fLC quotient 0.0994 and k-fLC index 18.15. Interestingly, the 25% (2/8) sensitivity in our cohort was showed by the k-fLC index 6.6 from metaanalysis of Leurs et al [17] and by Presslauer’s k-fLC index 5.9 [14]. This is concordant with the findings of Ferraro et al [20], who found similar k-fLC index 5.8 to be positive in 25% (23/92) of OCB negative MS patients. Although these diagnostic sensitivities are not fully satisfying, the detection of intrathecal immunoglobulin syntesis in 2 out of 8 OCB negative MS patients could be of diagnostic importance. The value of k-fLC markers in MS diagnostics is also supported by the findings of Goffette et al [22], who detected oligoclonal k-FLC bands to be positive in 54% (18/33) of OCB negative CSFs. The analysis of k-FLC using the isoelectric focusing is more difficult and time consuming compared to an automated k-FLC measurement. Although using polyacrylamide gel showed higher diagnostic sensitivity in MS than agarose gel (better resolution enabling detection of more oligoclonal k-FLC bands), both methods highly correlate and are suitable to detect intrathecal Ig synthesis in routine practice [23].
Conclusion
To our knowledge, this is the first study assessing the feasibility of novel k-fLC markers in a cohort of MS patients and controls from Slovakia. Excellent diagnostic accuracy of CSF k-fLC level, k-fLC quotient and k-fLC index was showed especially in the prediction of OCB positivity, where all novel marker’s AUC values were significantly higher than those of IgG index and IgGReiber, providing also much higher sensitivities and specificities. In MS diagnostics, the novel k-fLC markers also provided better diagnostic sensitivities and specificities than the IgG index and IgGReiber, but did not reach those of OCB detection. To the best of our knowledge, in the detection of intrathecal Ig synthesis, the practical use of k-fLC index or quotient seems to be more accurate than simple CSF k-fLC level measurement. Thus for now, to proove intrathecal Ig synthesis in patients with a suspected diagnosis of MS, we suggest a parallel use of k-fLC index and/or quotient and OCB examination. This is recommended especially in cases with equivocal or negative OCB findings. In the future, we plan further studies to validate diagnostic performance of k-fLC in larger cohorts of OCB negative MS patients.
Declaration of conflicting interests
The authors have no conflict of interest to declare with respect to the publication of this article.
Funding
This work was supported by the Slovak Scientific Grant Agency grants number VEGA 230/20 and VEGA 1/0266/18.
Ethical approval
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). The study was approved by the Ethics Committee of the Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin (Approval number: 1712/2015). All involved individuals gave a written informed consent with participation in the study.
Acknowledgements
The authors would like to thank the patients with multiple sclerosis and control individuals for their generous agreement to participate in this study. We also thank Miroslava Simekova and Ľubica Jesenska for technical assistance, and prim. Dr. Jozef Michalik for the help with patients’ stratification and biological samples management.
Contributorship
Daniel Cierny and Dusan Dobrota designed the study, Ema Kantorova and Egon Kurca were involved in patients’ recruitment, assessment of clinical diagnosis and samples collection, Maria Skerenova and Daniel Cierny performed the experiments and data analysis, Daniel Cierny wrote the manuscript draft, Jan Lehotsky and Dusan Dobrota critically reviewed the article. All authors contributed equally and approved the final version of the manuscript.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Prof. Dušan Dobrota, MD, CSc.
Department of Clinical Biochemistry
Jessenius Faculty of Medicine
in Martin
Comenius University in Bratislava
and University Hospital Martin
Kollárova 2, 036 01 Martin
Slovak Republic
e-mail: dusan.dobrota@uniba.sk
Accepted for review: 5. 10. 2020
Accepted for print: 15. 7. 2021
Zdroje
1. Thompson AJ, Banwell BL, Barkhof F et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17 (2): 162–173. doi: 10.1016/S1474-4422 (17) 30470-2.
2. Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001; 184 (2): 101–122. doi: 10.1016/s0022-510x (00) 00501-3.
3. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372 (9648): 1502–1517. doi: 10.1016/S0140-6736 (08) 61 620-7.
4. Bonnan M. Does disease-irrelevant intrathecal synthesis in multiple sclerosis make sense in the light of tertiary lymphoid organs? Front Neurol 2014; 5: 27. doi: 10.3389/fneur.2014.00027.
5. Freedman MS, Thompson EJ, Deisenhammer F et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol 2005; 62 (6): 865–870. doi: 10.1001/archneur.62.6.865.
6. Hegen H, Zinganell A, Auer M et al. The clinical significance of single or double bands in cerebrospinal fluid isoelectric focusing. A retrospective study and systematic review. PLoS One 2019; 14 (4): e0215410. doi: 10.1371/journal.pone.0215410.
7. Krakauer M, Schaldemose Nielsen H, Jensen J et al. Intrathecal synthesis of free immunoglobulin light chains in multiple sclerosis. Acta Neurol Scand 1998; 98 (3): 161–165. doi: 10.1111/j.1600-0404.1998.tb07287.x.
8. Bracco F, Gallo P, Menna R et al. Free light chains in the CSF in multiple sclerosis. J Neurol 1987; 234 (5): 303–307. doi: 10.1007/BF00314285.
9. Zeman D, Kušnierová P, Bartoš V et al. Quantitation of free light chains in the cerebrospinal fluid reliably predicts their intrathecal synthesis. Ann Clin Biochem 2016; 53 (Pt 1): 174–176. doi: 10.1177/0004563215579110.
11. Desplat-Jégo S, Feuillet L, Pelletier J et al. Quantification of immunoglobulin free light chains in cerebrospinal fluid by nephelometry. J Clin Immunol 2005; 25 (4): 338–345. doi: 10.1007/s10875-005-5371-9.
10. Hassan-Smith G, Durant L, Tsentemeidou A et al. High sensitivity and specificity of elevated cerebrospinal fluid kappa free light chains in suspected multiple sclerosis. J Neuroimmunol 2014; 276 (1–2): 175–179. doi: 10.1016/j.jneuroim.2014.08.003.
12. Pieri M, Storto M, Pignalosa S et al. KFLC Index utility in multiple sclerosis diagnosis: further confirmation. J Neuroimmunol 2017; 309: 31–33. doi: 10.1016/j.jneuroim.2017.05.007.
13. Senel M, Mojib-Yezdani F, Braisch U et al. CSF free light chains as a marker of intrathecal immunoglobulin synthesis in multiple sclerosis: a blood-CSF barrier related evaluation in a large cohort. Front Immunol 2019; 10: 641. doi: 10.3389/fimmu.2019.00641.
14. Presslauer S, Milosavljevic D, Brücke T et al. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J Neurol 2008; 255 (10): 1508–1514. doi: 10.1007/s00415-008-0954-z.
15. Duranti F, Pieri M, Centonze D et al. Determination of kFLC and k Index in cerebrospinal fluid: a valid alternative to assess intrathecal immunoglobulin synthesis. J Neuroimmunol 2013; 263 (1–2): 116–120. doi: 10.1016/j.jneuroim.2013.07.006.
16. Süße M, Hannich M, Petersmann A et al. Kappa free light chains in cerebrospinal fluid to identify patients with oligoclonal bands. Eur J Neurol 2018; 25 (9): 1134–1139. doi: 10.1111/ene.13667.
17. Leurs CE, Twaalfhoven H, Lissenberg-Witte BI et al. Kappa free light chains is a valid tool in the diagnostics of MS: a large multicenter study. Mult Scler 2020; 26 (8): 912–923. doi: 10.1177/1352458519845844.
18. Presslauer S, Milosavljevic D, Huebl W et al. Kappa free light chains: diagnostic and prognostic relevance in MS and CIS. PLoS One 2014; 9 (2): e89945. doi: 10.1371/journal.pone.0089945.
19. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44 (3): 837–845.
20. Ferraro D, Trovati A, Bedin R et al. Cerebrospinal fluid kappa and lambda free light chains in oligoclonal band-negative patients with suspected multiple sclerosis. Eur J Neurol 2020; 27 (3): 461–467. doi: 10.1111/ene. 14121.
21. Jagannath, S. Value of serum free light chain testing for the diagnosis and monitoring of monoclonal gammopathies in hematology. Clin Lymphoma Myeloma 2007; 7 (8): 518–523. doi: 10.3816/clm.2007.n.036.
22. Goffette S, Schluep M, Henry H et al. Detection of oligoclonal free kappa chains in the absence of oligoclonal IgG in the CSF of patients with suspected multiple sclerosis. J Neurol Neurosurg Psychiatry 2004; 75 (2): 308–310. doi: 10.1136/jnnp.2003.010710.
23. Zeman D, Kušnierová P, Hradílek P et al. Oligoklonální IgG a volné lehké řetězce – srovnání izoelektrické fokusace v agarózovém a polyakrylamidovém gelu. Cesk Slov Neurol N 2019; 82 (1): 68–75. doi: 10.14735/ amcsnn201968.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2021 Číslo 4
Nejčtenější v tomto čísle
- COVID-19 related olfactory impairment – diagnostics, significance and treatment
- Why do the nerve tracts decussate? Basic principles of the vertebrate brain organization
- CANVAS – a newly identified genetic cause of late-onset ataxia. Description of the first cases in the Czech Republic
- COVID-19 associated myelitis – a case report of rare complication of severe SARS-CoV-2 infection
