Skin grafting in surgical treatment of pressure ulcers
Transplantace kůže v chirurgické léčbě dekubitů
Základem léčby hlubokých dekubitů (III. a IV. kategorie) jsou různé chirurgické techniky provázené konzervativní terapií a preventivními opatřeními. Transplantace kůže představuje jednu z možností v chirurgické léčbě dekubitů, má však své specifické indikace. Cílem tohoto článku je tyto indikace v rámci plastické chirurgie objasnit a popsat nevýhody transplantace kůže v rekonstrukci dekubitů. Jedna z těchto indikací je prezentována v kazuistice 89leté pacientky s neklasifikovatelným dekubitem paty, který byl chirurgicky rekonstruován pomocí transplantace kůže.
Klíčová slova:
proleženina – kazuistika – decubitus – chirurgické rekonstrukční techniky – transplantace kůže
Authors:
A. Hokynková; P. Šín; T. Adlerová; F. Černoch
Authors‘ workplace:
Surgery, Faculty of Medicine, Masaryk
; University and University Hospital Brno
; Department of Burns and Plastic
Published in:
Cesk Slov Neurol N 2022; 85(Supplementum 1): 12-14
doi:
https://doi.org/10.48095/cccsnn2022S12
Overview
Optimal treatment of deep category of pressure ulcers/injuries (category III and IV) is represented by numerous surgical procedures accompanied with conservative therapy and preventative measures. Skin grafting represents a reconstructive option in plastic surgery with very specific indications in pressure ulcer‘s surgical therapy. The aim of this article is to clarify the indications for plastic surgery interventions and to describe possible disadvantages of skin grafting in pressure ulcers reconstruction. One of the specific indications is presented as case report of 89-years-old women with unstageable pressure ulcer in heel area that was surgically reconstructed by skin grafting.
Keywords:
Skin grafting – pressure ulcer – case report – pressure injury – reconstructive surgical procedures
Introduction
Optimal treatment of deep category of pressure ulcers (PUs) /injuries (category III and IV) is represented by numerous surgical procedures accompanied with conservative therapy and preventive care. Surgical treatment of these types of PUs usually consists of two steps: debridement and proper reconstructive surgery of the wound [1]. Generally, well known reconstructive ladder – wound closure from the easiest reconstructive technique to the most demanding one, is usually used in surgical coverage of most majority wounds in plastic surgery [2–5]. However, in such specific type of defects as the PUs really are, the reconstructive ladder is quite modified. Healing by secondary intention can be expected primarily in minor defects. It is applicable also in bigger defects, in case when surgical treatment is not recommended for some reasons (poor general health status, inability to undergo general anaesthesia etc.). In these cases, conservative therapy can lead to healing up of these defects, but it may take several months. Second stair in the reconstructive ladder is primary closure of the defects. Nevertheless, direct suture in PUs surgical therapy is recommended only in certain indications [6], especially in small sized defect. Disadvantage of this technique is represented by the fact that resulting scar is localised above the bony prominence. Connective tissue of the scar is less resistant against the pressure than other tissues (fascia, muscle etc.) According to our experience, there is a higher risk of dehiscence development [7] and recurrence of PUs using direct suture in comparison with reconstructions using flap closure technique. Tissue expansion [8] and free flap transfer are on the top of the reconstructive ladder techniques, but they are used in PUs reconstructive procedures only rarely. Therefore, fasciocutaneous or musculocutaneous flaps [9], that are widely used in surgical treatment of PUs, are rightly signed as a workhorse in reconstructions of Pus [10].
Skin grafting technique description and indications in PUs therapy
Third stair in reconstructive ladder in plastic surgery belongs to skin grafting (SG). This method is generally used in burns, extensive soft tissue defects, or specific defects localised in the head or another area. Skin grafting can be performed in a form of split-thickness skin graft (STSG) that contains the epidermis and a part of the dermis, or in a form of full--thickness skin graft (FTSG) containing the epidermis and the entire dermis. STSG is usually harvested by airdermatom from the ventral thigh area and then meshed in ratio of 1: 1.5, 1.2, 1: 3. FTSG is often harvested from the retroauricular, supraclavicular or groin area and is perforated only. FTSG is performed in areas where the scar contraction is expected or in reconstruction of head area. Fixation to the defects is performed by skin stapler or by non-resorbable filament. Basic healing up is around 7–10 days and continues with scar maturation. This method has some disadvantages, therefore its using in reconstruction of PUs is very restricted. PUs are classified as complicated healing wounds or non-healing wounds. These types of wounds are burdened almost always by polymicrobial wound bed colonisation, often with resistant strains (Methicillin resistant Staphylococcus aureus etc.). Skin grafting as a method of choice has to be taken in consideration only in case when the wound bed is minimally colonised by bacteria. Local bacterial infection leads to damage of skin graft. On the other hand, the donor site has to be protected from transposed infection from the wound bed of PUs. STSG is very thin (0.008–0.75mm) [11]) and unpliable and has to be placed on the shallow defects with well-granulating tissue refined of avital tissue and slough [12]. Skin grafting can be used only in category III, not in category IV of PUs, where bone is exposed or affected by lysis or osteomyelitis. Another disadvantage of this reconstructive method is the fragility of the skin graft. It must be considered that in patient suffering from PU, the main cause-pressure between bony prominences and the base, as well as friction shear, will continue. Therefore, SG is not recommended as a method of choice in PUs reconstruction. However, in certain indications this technique may be very useful. It includes reconstructions of small or moderate PUs in head area, incidence of which was rising up during COVID-19 pandemic, especially in patients ventilated in the prone position. Head area is well-vascularised, thus providing adequate wound bed for skin grafting. SG can be considered as a reconstructive option in medical-device-related PUs, especially in reconstruction of larger defects of mucosal membrane PUs [13], according to localisation, size or wound bed. Other indication is reconstruction of extensive soft tissue defects, for example positional trauma, where local flaps are insufficient for covering. Another possibility to use this technique is PUs localised in lower limb, especially in heel area, as described below in the case report.
Case report
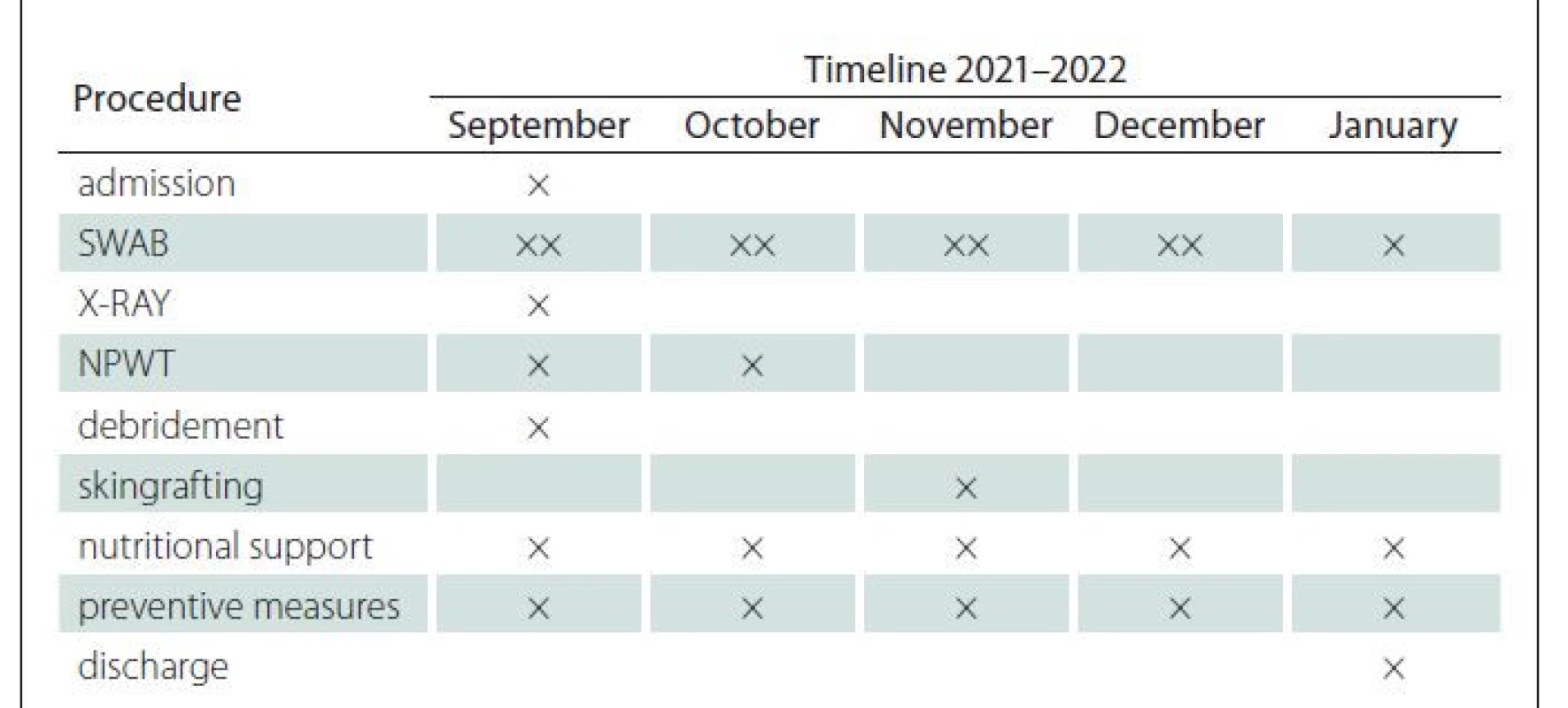
Eighty-nine years old woman with severe comorbidities (hypertension, diabetes mellitus, post stroke syndrom, immobility etc.) was admitted for PU localised on the left heel, classified as hospital acquired PUs. The PUs was clinically expressed as black thick eschar on the surface with slushy smelling and movable base accompanied by marginal erythema of surrounding skin (Fig. 1, 2). The size of this unstageable PU was 8 x 7 cm with unknown depth. It was indicated for surgical treatment due to suspicion of local infection of soft tissue. The X-ray was performed to rule out osteomyelititis or osteolysis of calcaneus. Sharp debridement was performed in spinal anaesthesia with removing all the avital tissue and slough, including necrotic parts of plantar fascia and insertion of flexor digitorum brevis et longus muscle. After the surgery, PU was classified to a category IV – exposed, but stiff calcaneal bone in size 2 x 1 cm. Negative pressure wound therapy (NPWT) using hydrochlorohexidin dressing supplying foam was used to support growth of granulation tissue, especially above the exposed bone part (Fig. 3). Due to worsening of general health status (hypertension decompensation, infection in the urinary tract and the inability to undergo general anaesthesia) reconstruction using flap reconstructive surgery was not indicated. Therefore, conservative therapy (wet dressing) was running with regularly monitoring of bacterial wound bed contamination (Proteus mirabilis, Prevotella melanogenica, Enterobacter cloace ESBL-extended spectrum beta-lactamase, Pseudomonas aeruginosa) and accurate antibiotics therapy for next 2 months. The granulation tissue in the wound bed grew up and covered exposed bone. Wound was microscopically sterile before reconstructive surgery. Due to general health status, SG in local anaesthesia was performed. STSG was harvested from ventral part of the left thigh, meshed in ratio 1: 1.5 and fixed into the wound with bolus of gauze. Secondary local infection of the donor site occurred (and treated by conservative therapy) postoperatively. Skin graft was healed up in majority of the wound in 14 days, but two months after the surgery small rest defect remains (Fig. 4.) treated by conservative therapy. Diagnostic and treatment timeline is shown in the Tab. 1.
Obr. 1. Neklasifi kovatelný dekubitus levé paty.

Obr. 2. Detailní obraz eschary.

Obr. 3. Granulační tkáň and patní kostí.

Obr. 4. Zbytkový defect v oblasti levé paty – 2 měsíce po rekonstrukci
dermoepidermálním kožním štěpem.

NPWT – negative pressure wound therapy
Conclusion
Skin grafting is not a method of choice in reconstruction of pressure ulcers, but it still has its own place in pressure ulcer‘s surgery treatment in specific indications.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Petr Šín, MD, PhD.
Department of Burns and Plastic
Surgery
Faculty of Medicine,
Masaryk University
and University Hospital
Jihlavská 20
625 00 Brno
e-mail: p.sin@seznam.cz
Sources
1. Hokynková A, Šín P, Černoch F et al. Employment of flap surgery in pressure ulcers surgical treatment. Cesk Slov Neurol N 2017; 80 (Supp1): S41–S44. doi: 10.14735/amcsnn2017S41.
2. Wong CJ, Niranjan N. Reconstructive stages as an alternative to the reconstructive ladder. Plast Reconstr Surg 2008; 121 (5): 362e–363e. doi: 10.1097/PRS.0b013e31816b1171.
3. Mardini S, Wei FC, Salgado CJ et al. Reconstruction of the reconstructive ladder. Plast Reconstr Surg 2005; 115 (7): 2174. doi: 10.1097/01.prs.0000165497.92397.be.
4. Gottlieb LJ, Krieger LM. From the reconstructive ladder to the reconstructive elevator. Plast Reconstr Surg 1994; 93 (7): 1503–1504. doi: 10.1097/00006534-199406000-00027.
5. Rubayi R, Wagenheim BR, Mcleland A. Reconstructive plastic surgery of pressure ulcers. Berlin: Springer 2015.
6. Bergstrom N, Bennett MA, Carlson CE et al. Treatment of pressure ulcers. Clinical practice guideline #15. Rockville, MD: US Dept of Health and Human Services’, Public Health Service, Agency for Health Care Policy and Research, 1994.
7. Anthony JP, Huntsman WT, Mathes SJ. Changing trends in the management of pelvic pressure ulcers: a 12-year review. Decubitus 1992; 5 (3): 44–51.
8. Esposito G, Di Caprio G, Ziccardi P et al. Tissue expansion in the treatment of pressure ulcers. Plast Reconstr Surg 1991; 87 (3): 501–508. doi: 10.1097/00006534-199103000-00018.
9. Černoch F, Jelínková Z, Rotschein P. Reconstruction of recurrent ischiadic pressure ulcer using turnover hamstring muscle flap. Cesk Slov Neurol N 2019; 82 (Supp1): S15–S18. doi: 10.14735/amcsnn2019S15.
10. Chen YC, Huang EY, Lin PY. Comparison of gluteal perforator flaps and gluteal fasciocutaneous rotation flaps for reconstruction of sacral pressure sores. J Plast Reconstr Aesthet Surg 2014; 67 (3): 377–382. doi: 10.1016/j.bjps.2013.12.029.
11. Gupta DK. Thin and ultra thin split thickness skin grafts (STSG-UT, STSG-T): In: Microskin grafting for vitiligo. London: Springer 2009: 15–18. doi: 10.1007/978-1-84882-605-2_5.
12. Sørensen JL, Jørgensen B, Gottrup F. Surgical treatment of pressure ulcers. Am J Surg 2004; 188 (1A Suppl): 42–51. doi: 10.1016/S0002-9610 (03) 00290-3.
13. Hess CT. Classification of pressure injuries. Adv Skin Wound Care 2020; 33 (10): 558–559. doi: 10.1097/ 01.ASW.0000697324.90597.6d.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

Most read in this issue
- Monitoring the prevalence of pressure ulcers – a comparison of national data with data of a specific health care provider – University Hospital Ostrava
- Nurses‘ knowledge in the field of specific prevention and treatment of heels pressure injuries
- Standardization of wound care for patients in Austria, Germany and Slovakia
- Advanced practice nursing in the field of wound management
