Effects of fluoxetine on the restoration of functional independence in patients after acute cerebral infarction and prognostic factors
Vliv fluoxetinu na obnovu funkční nezávislosti u pacientů po akutní ischemické cévní mozkové příhodě a prognostické faktory
Cíl: Cílem této studie bylo prozkoumat vliv fluoxetinu na obnovu funkční nezávislosti po akutní ischemické CMP (iCMP) a faktory ovlivňující prognózu. Pacienti a metody: Jedná se o retrospektivní studii na 196 pacientech po iCMP, kteří podstoupili léčbu od května 2018 do září 2020. Pacienti byli pomocí tabulky náhodných číslem rozděleni do kontrolní a experimentální skupiny (n = 98 v každé skupině). Kontrolní skupině byly podávány rutinní medikamenty a experimentální skupině také fluoxetin po dobu 60 dní. Zároveň obě skupiny podstupovaly rehabilitační trénink. Jejich neurologické funkce, míra deprese, motorika končetin a aktivity běžného života (activities of daily living; ADL) byly hodnoceny pomocí škály National Institutes of Health Stroke Scale (NIHSS), Hamiltonovy škály pro hodnocení deprese (Hamilton Rating Scale for Depression; HAMD), Fugl-Meyerovy hodnotící stupnice (Fugl-Meyer Assessment; FMA) a indexu Barthelové. Analyzovány byly nezávislé rizikové faktory špatné prognózy pacientů po iCMP. Výsledky: Po dokončení léčby bylo v porovnání s výsledky skupin před léčbou zjištěno významné zvýšení skóre FMA a ADL, NIHSS a HAMD skóre se naopak u obou skupin snížilo (p < 0,05). U experimentální skupiny došlo v porovnání s kontrolní skupinou k významně lepším výsledkům léčby i zlepšení motorických a neurologických funkcí (p < 0,05). Nezávislými rizikovými faktory špatné prognózy (p < 0,05) byly objem infarktu, výchozí skóre NIHSS, diabetes mellitus, arteriální hypertenze, leukoaraióza, hyperurikémie a infekce. Závěr: Fluoxetin může významně zlepšit výsledky léčby i motorické a neurologické funkce pacientů po iCMP. Velký objem infarktu, vysoké výchozí skóre NIHSS, diabetes mellitus, arteriální hypertenze, leukoaraióza, hyperurikémie a infekce zvyšují riziko špatné prognózy.
Klíčová slova:
prognóza – ischemická cévní mozková příhoda – fluoxetin – poruchy motoriky – neurologické symptomy
Authors:
X. Wu 1; Y. Xiao 1; H. Zhang 2
Authors‘ workplace:
Department of Medical Rehabilitation, Huizhou Central People‘s, Hospital, Huizhou, China
1; Department of Medical Rehabilitation, Affi liated Hospital and Second, Clinical Medical College of North, Sichuan Medical University, Nanchong, Central Hospital, Nanchong, China
2
Published in:
Cesk Slov Neurol N 2022; 85(1): 70-75
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn202270
Overview
Aim: The aim of this study was to explore the effects of fluoxetine on the restoration of functional independence in patients after acute cerebral infarction (ACI) and the factors influencing the prognosis. Patients and methods: This was a retrospective study including 196 ACI patients treated from May 2018 to September 2020. They were divided into control and experimental groups (N = 98 in each group) using a random number table. The control group was treated with routine medication and the experimental group was given also fluoxetine for 60 days. Meanwhile, both groups were subjected to rehabilitation training. Their neurological function, depression, limb motor function and activities of daily living (ADL) were assessed using the National Institutes of Health Stroke Scale (NIHSS), Hamilton Rating Scale for Depression (HAMD), Fugl-Meyer Assessment (FMA) and Barthel index, respectively. The independent risk factors for poor prognosis of ACI patients were analyzed. Results: After treatment, the FMA and ADL scores rose significantly, while the NIHSS and HAMD scores declined in both groups compared with those before treatment (P < 0.05). Compared with the control group, the experimental group had significantly improved treatment outcomes as well as motor and neurological functions (P < 0.05). Infarction volume, baseline NIHSS score, diabetes mellitus, arterial hypertension, leukoaraiosis, hyperuricemia and infection were independent risk factors for poor prognosis (P < 0.05). Conclusion: Fluoxetine can significantly improve the treatment outcomes as well as the motor and neurological functions of patients with ACI. Large infarction volume, high baseline NIHSS score, diabetes mellitus, arterial hypertension, leukoaraiosis, hyperuricemia and infection increase the risk of poor prognosis.
Keywords:
Prognosis – cerebral infarction – fluoxetine – motor disorders – neurological symptoms
Introduction
Acute cerebral infarction (ACI), which is caused by the vessel stenosis or occlusion due to local cerebrovascular atherosclerotic plaques or embolism [1,2], has high rates of morbidity, disability and mortality [3]. Currently, intravenous thrombolysis and mechanical recanalization are being generally used in the clinical practice for the recanalization of the occluded vessels, restoration of the blood flow and improvement of the prognosis of ACI patients [4]. Early rehabilitation training is of great importance for restoration of neurological functions and reduction of complications.
As a selective serotonin reuptake inhibitor, fluoxetine can increase brain-derived neurotrophic factors in ACI patients by blocking serotonin reuptake, thereby exerting an antidepressant effect [5,6]. Therefore, we herein explored the effects of fluoxetine on the motor and neurological functions of patients with ACI and the factors influencing the prognosis, aiming to provide a theoretical basis for clinical treatment and prognostic evaluation.
Patients and methods
Inclusion and exclusion criteria
Inclusion criteria were: (1) patients meeting the diagnostic criteria for ACI [7] and diagnosed as cerebral infarction using the brain CT or MRI; (2) with first-onset ACI and time of onset < 24 h; and (3) without undergoing other treatments before the admission.
Exclusion criteria were: (1) patients with cerebral hemorrhage or transient ischemic attack; (2) with malignancies; (3) with severe liver or kidney dysfunction; (4) with a previous history of myocardial infarction or stroke; or (5) with coagulation dysfunction.
Methods
The control group was routinely given aspirin (100 mg/day), clopidogrel (75 mg/day) and atorvastatin (10 mg/day) for 60 days. Beside the standard treatment, the experimental group was orally administered also fluoxetine hydrochloride (Patheon, Champaret, France) 20 mg/day after the meal every morning for 60 days.
Meanwhile, both groups were subjected to rehabilitation training during 60 days.
1) Regular posture changes: patients were turned over once every 2 h.
2) Correct posture was maintained to prevent abnormal movements or contractures and joint deformation of the affected limbs. In the supine position, the shoulders were abduced by 50° and internally rotated by 50°, and the elbows were flexed by 40–50° and externally rotated using soft cushions. The hip bone was moved forward, and the lower limbs were internally rotated with soft pillows outside the legs. The feet were placed at right angles to the bed with footrests to prevent them from sagging or inversion. The pressure and time of lying on the affected limbs were reduced.
3) Joint range of movement and muscle strength training: passive exercise was gradually changed to the assisted passive exercise and then to the active exercise. Each joint was trained twice daily, 20 times during each training.
4) Level III balance training from a sitting to a standing position was conducted sequentially.
5) Gait training.
6) Stair-climbing training.
7) Training of activities of daily living (ADL).
Observation indices
Neurological function was the primary observation index [8]. The degree of neurological impairment was assessed using the National Institutes of Health Stroke Scale (NIHSS). The higher score means more severe neurological impairment.
Secondary observation indices were:
1) depressive symptoms [9] – the depression was assessed using the Hamilton Rating Scale for Depression (HAMD). A higher score means more severe depressive symptoms.
2) Limb motor function [10] was evaluated using the Fugl-Meyer Assessment (FMA) (66 points for the upper limbs and 34 points for the lower limbs). A higher score means better motor function.
3) ADL [11] were assessed with the Barthel index. The total score is 100 points, and a higher score indicates better ADL.
Tertiary observation indices were: adverse reactions during treatment, including skin rash, mildly elevated transaminase level, transient gastrointestinal discomfort, mental disorders, partial epilepsy, hyponatremia and insomnia.
Assessment criteria for treatment outcomes [12]
Cured: The NIHSS score declined by more than 90%, infarct lesions disappeared, and disability reached grade 0. Markedly effective: The NIHSS score declined by 46–89%, infarct lesions decreased, and disability reaching grades 1–3. Effective: The NIHSS score declined by 18–45% and infarct lesions decreased. Ineffective: The NIHSS score decreased or increased by less than 18% and infarct lesions remained unchanged or became larger. Total response rate = (effective cases + markedly effective cases + cured cases) /total cases × 100%.
Follow-up
The experimental group was followed up for 6 months by telephone calls, outpatient visits and medical history enquiries. The patients’ prognosis was assessed using the Glasgow Outcome Scale (GOS) [13]. According to the GOS score, the patients were divided into the good prognosis group (GOS score of ≥ 4 points) and the poor prognosis group (GOS score of < 4 points).
Statistical analysis
The sample size was calculated according to the two independent-sample test equations
where N is the number of required cases, Za/2 is the Z value corresponding to a = 0.05 (which is 1.96), Zb is the Z value corresponding to the type II error probability b (b = 0.20, so Zb = 0.84), and d is the allowable error (which is generally 0.6). As a result, N was calculated as 87, i.e., at least 87 cases should be included in each group. Finally, 98 cases were included in each group.
SPSS 23.0 software (IBM, Armonk, NY, USA) was used for statistical analysis. Measurement data were expressed as mean ± standard deviation (c–± s), and compared by the t test between two groups. Numerical data were expressed as a percentage (%), and compared by the c2 test between two groups. The influencing factors for the prognosis of ACI patients were explored by multivariate logistic regression analysis. P < 0.05 was considered to be statistically significant.
Results
This was a retrospective study including 196 ACI patients treated in our hospital from May 2018 to September 2020. There were 114 males and 82 females aged 45–78 (average age 60.25 ± 9.55) years. They were divided into control and experimental groups (N = 98 in each group) using a random number table. Baseline data of the control group and the experimental group are shown in Tab. 1. No significant differences were found in the average age, sex ratio, type of cerebral infarction, area of cerebral infarction and time of onset between the two groups (P > 0.05 in all cases).
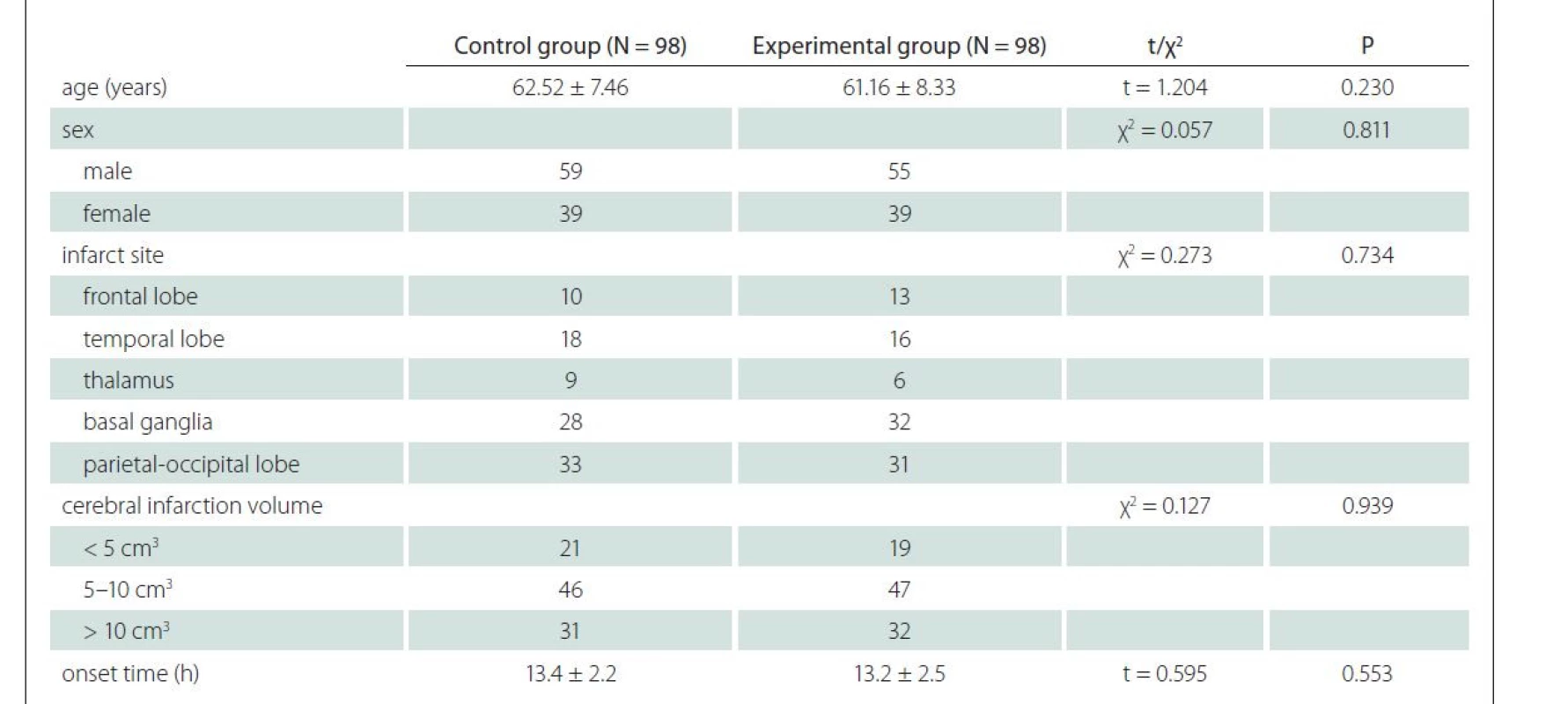
Before treatment, there were no significant differences in the FMA score (upper limb, lower limb and total scores) and the ADL score between the control and experimental groups (P > 0.05 in all cases). After treatment, the FMA and ADL scores rose significantly in both groups compared with those before treatment (P < 0.05) (Tab. 2).
ADL – activities of daily living; FMA – Fugl-Meyer Assessment; N – number
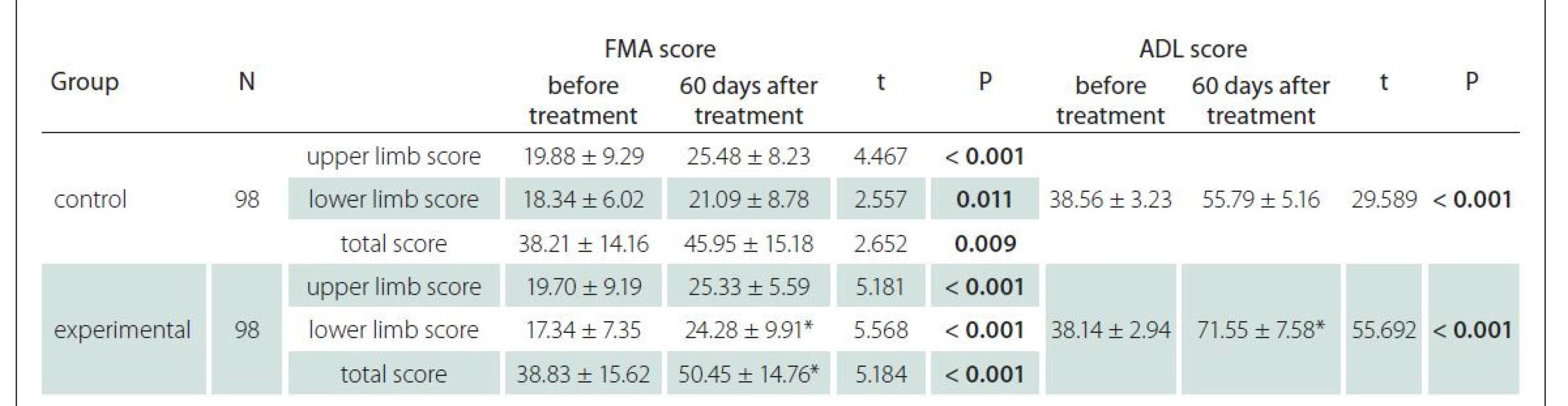
Before treatment, there were no significant differences in the NIHSS and HAMD scores between the control and experimental groups (P > 0.05 in both cases). After treatment, both the NIHSS and HAMD scores declined significantly in the two groups and the outcomes of the experimental group were better than those of the control group (P < 0.05) (Tab. 3).
HAMD – Hamilton Rating Scale for Depression; N – number; NIHSS – National Institutes of Health Stroke Scale
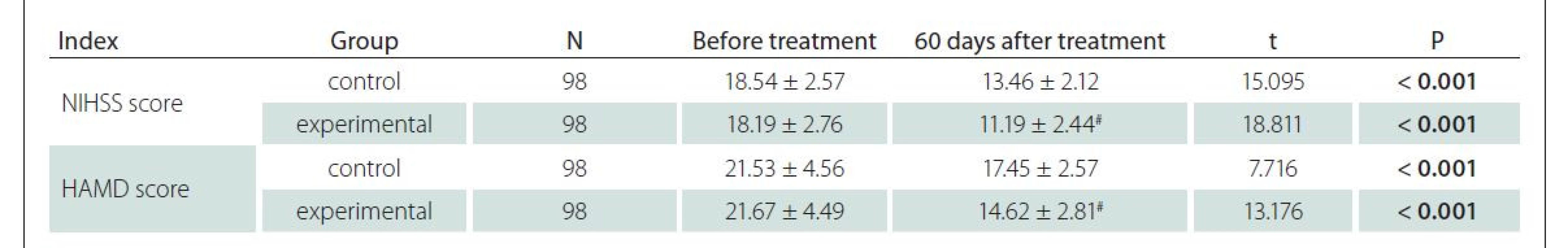
The total response rate of the experimental group was significantly higher than that of the control group (P < 0.05) (Tab. 4).
After treatment, the incidence rates of adverse reactions such as rash, mild transaminase elevation, transient gastrointestinal discomfort, mental disorders, partial epilepsy, hyponatremia and insomnia showed no significant difference between the two groups (P > 0.05 in all cases) (Tab. 5).
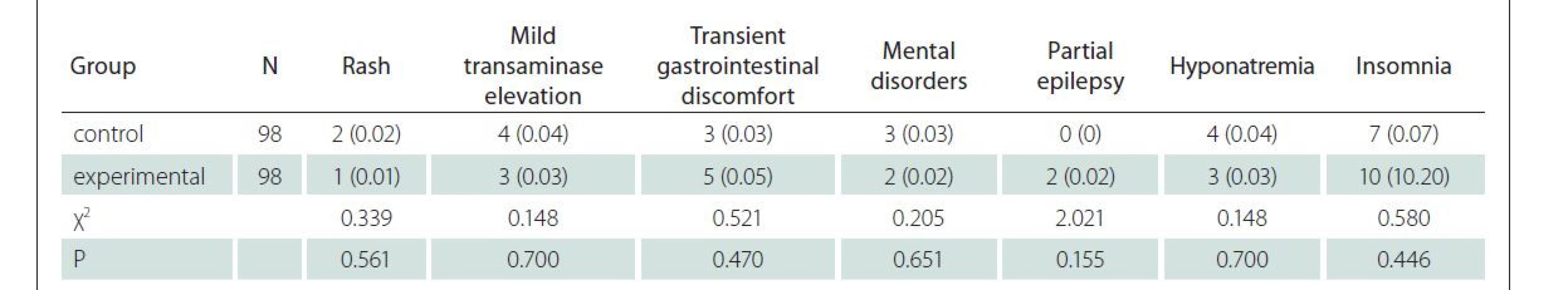
In the experimental group, 59 and 39 cases had a good and a poor prognosis, respectively. Two patients died due to the extensive cerebral embolism and intracranial hemorrhage. Using the univariate analysis, infarction volume, baseline NIHSS score, arterial hypertension, hyperlipidemia, leukoaraiosis, hyperuricemia, atrial fibrillation and infection were identified as factors influencing the prognosis in the experimental group (P < 0.05 in all cases) (Tab. 6).
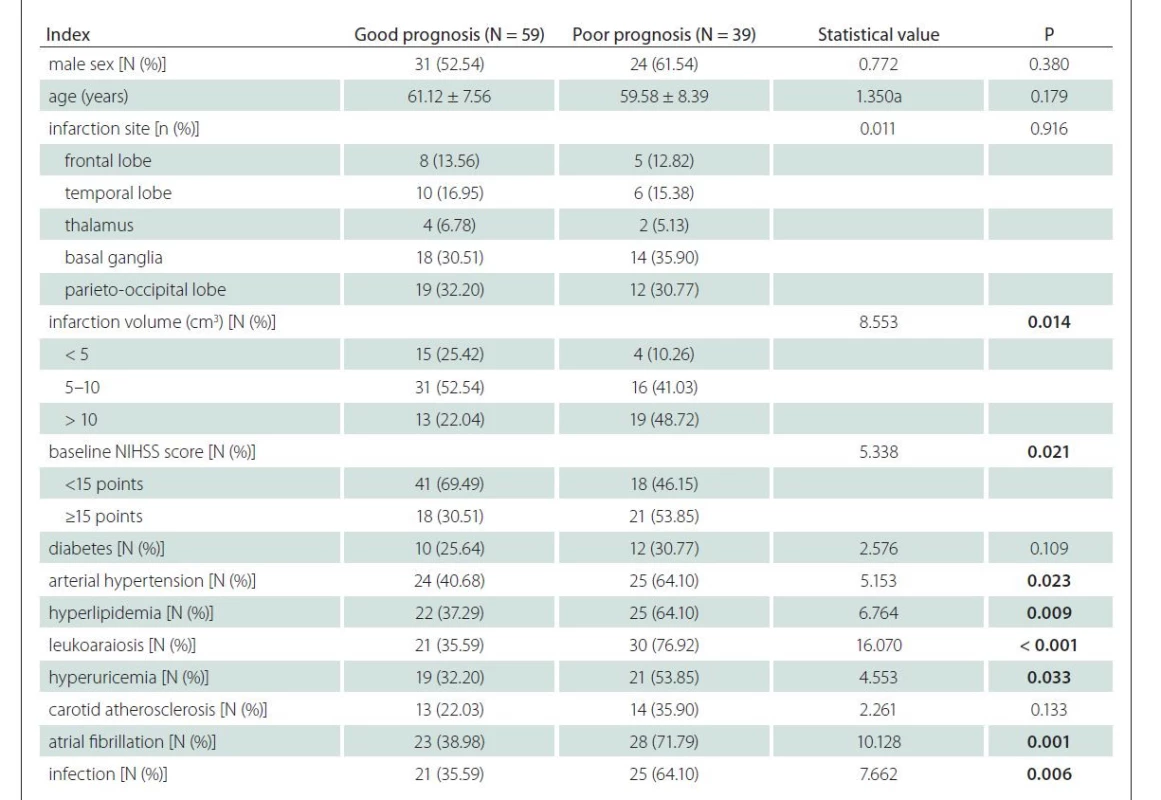
N – number; NIHSS – National Institutes of Health Stroke Scale
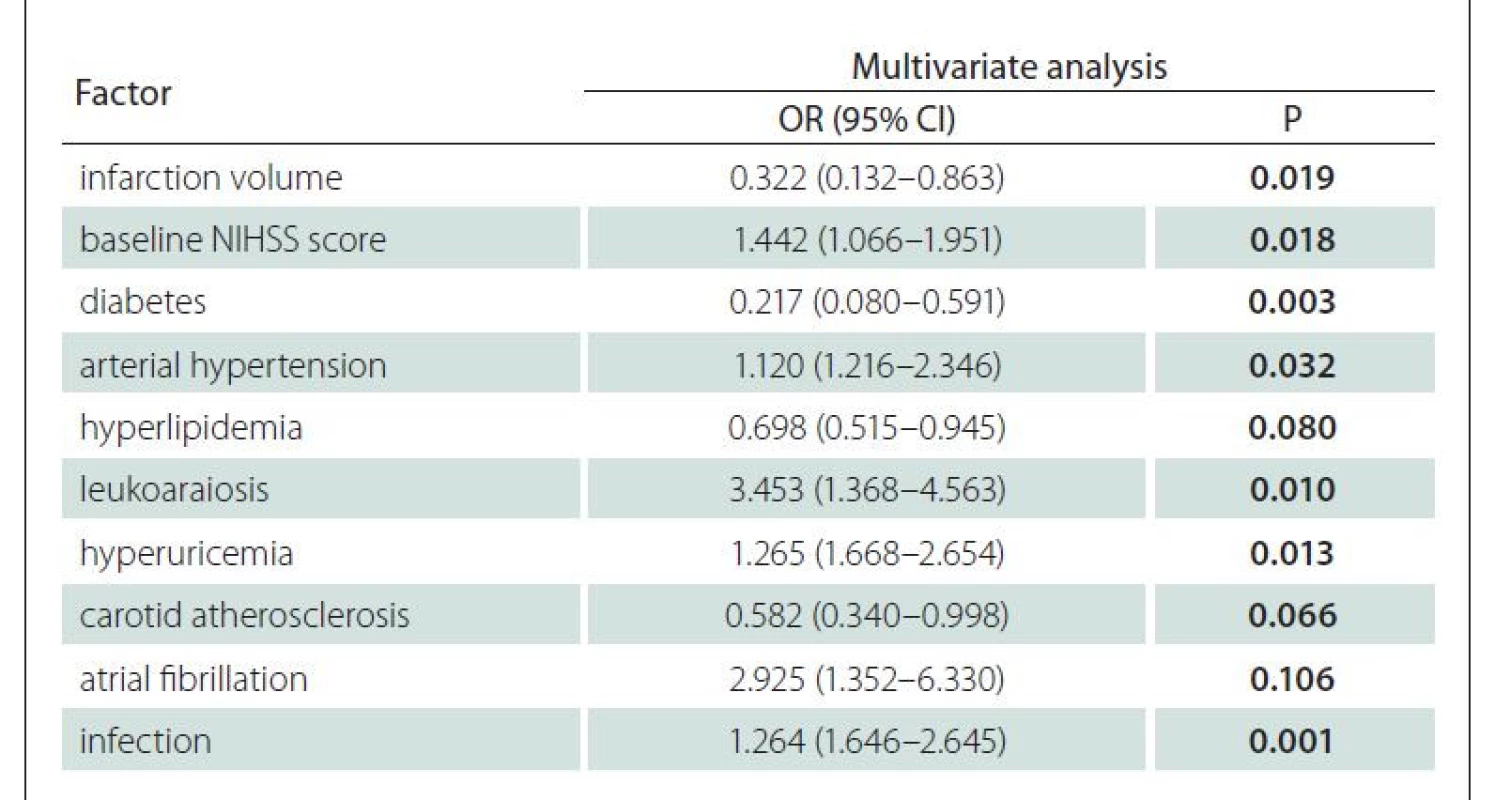
Multivariate regression analysis was conducted using the prognosis of the experimental group as the dependent variable, and factors with statistical significance in univariate analysis as the independent variables. The results showed that infarction volume, baseline NIHSS score, diabetes mellitus, arterial hypertension, leukoaraiosis, hyperuricemia and infection were independent risk factors for the poor prognosis in the ACI patients (P < 0.05) (Tab. 7).
Discussion
Acute cerebral infartion has high disability and mortality rates. Patients with arterial hypertension and atherosclerosis and those over 65 years of age represent high-risk groups for ACI [14,15]. Currently, ACI is treated mainly using the intravenous thrombolysis and mechanical recanalization in the clinical practice. Due to different degrees of neurological damage, however, patients suffer from sequelae such as aphasia, memory loss, limb paralysis and depression after treatment, so rehabilitation training and drug therapy are usually needed [16]. Fluoxetine is a highly selective serotonin uptake inhibitor able to enhance the serotonin transmission function by increasing the serotonin level in the brain and those of brain-derived neurotrophic factors, thereby exerting an antidepressant effect [17]. It has been confirmed [18] that rehabilitation training can effectively improve the neurological and motor functions and the prognosis of patients with ACI. Notably, earlier rehabilitation training is more beneficial. In the present study, therefore, fluoxetine was used for intervention starting in early rehabilitation training, with routine medication as the control. After treatment, the FMA and ADL scores rose significantly, while the NIHSS and HAMD scores declined in the control and experimental groups compared with those before treatment. The treatment outcomes of the experimental group were superior to those of the control group, and the two groups had similar incidence rates of adverse reactions such as rash, transient gastrointestinal discomfort, mental disorders and insomnia, indicating that early fluoxetine use is safe and effective is ACI. Bertani et al [19] reported that rehabilitation training promoted the formation of new synapses and nerve pathways in the nervous system, restored the neurological functions and reduced the disability rate. Antidepressant drugs can regulate neurotransmitters and restore their balance, thereby relieving the mental stress in ACI patients and improving their enthusiasm during rehabilitation training.
Herein, the results of multivariate analysis revealed that infarction volume, baseline NIHSS score, diabetes mellitus, arterial hypertension, leukoaraiosis, hyperuricemia and infection were independent risk factors for the poor prognosis of patients with ACI. Wang et al [20] showed that a larger infarction volume was more disadvantageous to the rescue of patients with ischemic penumbra as an independent risk factor for the short-term prognosis of ACI patients. The NIHSS score reflects the severity of cerebral infarction and a higher baseline NIHSS score corresponds to more severe neurological impairment, which is not conducive to the prognosis of patients [21]. Diabetes mellitus leads to metabolic disorders and arterial hypertension increases the risk of subcortical and periventricular white matter lesions, both of which can cause brain damage [22]. The degree of leukoaraiosis in patients with ACI has recently been positively correlated with the risk of hemorrhagic transformation, probably because leukoaraiosis and recombinant tissue plasminogen activator cooperate to aggravate endothelial function damage [23]. Lin et al [24] found that hyperuricemia easily induced the generation of free radicals in vivo, leading to neurological impairment and cognitive dysfunction in patients. Infection easily elevates the body temperature and accelerates the formation of necrotic tissues in the ischemic penumbra, resulting in the poor prognosis of patients with ACI.
In conclusion, fluoxetine can significantly improve the treatment outcomes as well as the motor and neurological functions of patients with ACI. Larger infarction volume, higher baseline NIHSS score, diabetes mellitus, arterial hypertension, leukoaraiosis, hyperuricemia and infection indicate a higher risk of poor prognosis of ACI patients. It is necessary to combine this therapy with reasonable intervention measures for the reduction of the risk of poor prognosis.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). This study has been approved by the ethic committee of Nanchong Central Hospital and written informed consent has been obtained from all patients.
Conflict of interest
The authors declare that they have no potential conflicts of interest concerning drugs, products or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Han Zhang
Department of Medical
Rehabilitation
Affi liated Hospital and Second
Clinical Medical College of North
Sichuan Medical University
Nanchong Central Hospital
Nanchong 637000
Sichuan Province
China
e-mail: quebersaipaa1973@web.de
Accepted for review: 2. 11. 2021
Accepted for print: 8. 2. 2022
Sources
1. Nakamura Y, Nakajima H, Kimura F et al. Preventive effect of cilostazol on pneumonia in patients with acute cerebral infarction. J Stroke Cerebrovasc Dis 2018; 27 (9): 2354–2359. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.024.
2. Ke Z, Zhao Y, Wang C et al. The alliance with expanding blood volume and correcting anemia is an effective therapeutic measure for the adult anemia patients of acute cerebral infarction. Int J Neurosci 2018; 128 (5): 429–434. doi: 10.1080/00207454.2017.1393419.
3. Xu X, Li C, Wan T et al. Risk factors for hemorrhagic transformation after intravenous thrombolysis in acute cerebral infarction: a retrospective single-center study. World Neurosurg 2017; 101: 155–160. doi: 10.1016/j.wneu.2017.01.091.
4. Lee XR, Xiang GL. Effects of edaravone, the free radical scavenger, on outcomes in acute cerebral infarction patients treated with ultra-early thrombolysis of recombinant tissue plasminogen activator. Clin Neurol Neurosurg 2018; 167: 157–161. doi: 10.1016/j.clineuro.2018.02. 026.
5. Pariente J, Loubinoux I, Carel C et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol 2001; 50 (6): 718–729. doi: 10.1002/ana.1257.
6. Chavda N, Kantharia ND, Jaykaran. Effects of fluoxetine and escitalopram on C-reactive protein in patients of depression. J Pharmacol Pharmacother 2011; 2 (1): 11–16. doi: 10.4103/0976-500X.77091.
7. Gao CY, Zhang XJ. Guidelines for the diagnosis and treatment of Chinese cerebral infarction with integrated traditional Chinese and western medicine. Chin J Integr Tradition West Med 2018; 38: 136–144.
8. Huang P, He XY, Xu M. Effect of Argatroban injection on clinical efficacy in patients with acute cerebral infarction: preliminary findings. Eur Neurol 2021; 84 (1): 38–42. doi: 10.1159/000512813.
9. Williams JB, Kobak KA, Bech P et al. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol 2008; 23 (3): 120–129. doi: 10.1097/YIC.0b013e3282f948f5.
10. Song X, Chen S, Jia J et al. Cellphone-based automated fugl-meyer assessment to evaluate upper extremity motor function after stroke. IEEE Trans Neural Syst Rehabil Eng 2019; 27 (10): 2186–2195. doi: 10.1109/ TNSRE.2019.2939587.
11. Mlinac ME, Feng MC. Assessment of activities of daily living, self-care, and independence. Arch Clin Neuropsychol 2016; 31 (6): 506–516. doi: 10.1093/arclin/acw 049.
12. Wang CQ. Effect of early rehabilitation training on motor function and the level of serum brain-derived neurotrophic factor in patients with acute cerebral infarction. J Xinxiang Med Uni 2017; 34: 946–948.
13. Yamal JM, Hannay HJ, Gopinath S et al. Glasgow Outcome Scale measures and impact on analysis and results of a randomized clinical trial of severe traumatic brain injury. J Neurotrauma 2019; 36 (17): 2484–2492. doi: 10.1089/neu.2018.5939.
14. Zhang H, Li CL, Wan F et al. Efficacy of cattle encephalon glycoside and ignotin in patients with acute cerebral infarction: a randomized, double-blind, parallel-group, placebo-controlled study. Neural Regen Res 2020; 15 (7): 1266–1273. doi: 10.4103/1673-5374.27 2616.
15. Li XX, Liu SH, Zhuang SJ et al. Effects of intravenous thrombolysis with alteplase combined with edaravone on cerebral hemodynamics and T lymphocyte level in patients with acute cerebral infarction. Medicine (Baltimore) 2020; 99: e23414. doi: 10.1097/MD.0000 000000023414.
16. Zhao YJ, Nai Y, Ma QS et al. Dl-3-n-butylphthalide protects the blood brain barrier of cerebral infarction by activating the Nrf-2/HO-1 signaling pathway in mice. Eur Rev Med Pharmacol Sci 2018; 22 (7): 2109–2118. doi: 10.26355/eurrev_201804_14744.
17. Feng BL, Wang QC, Li ZY. Influence of Jieyu Huoxue Decoction on rehabilitation of patients with depression after cerebral infarction. Zhong Xi Yi Jie He Xue Bao 2004; 2 (3): 182–184. doi: 10.3736/jcim20040309.
18. Lo WL, Mao YR, Li L et al. Prospective clinical study of rehabilitation interventions with multisensory interactive training in patients with cerebral infarction: study protocol for a randomised controlled trial. Trials 2017; 18 (1): 173. doi: 10.1186/s13063-017-1874-y.
19. Bertani R, Melegari C, De Cola MC et al. Effects of robot-assisted upper limb rehabilitation in stroke patients: a systematic review with meta-analysis. Neurol Sci 2017; 38 (9): 1561–1569. doi: 10.1007/s10072-017-2995-5.
20. Wang J, Chen J, Xiong XY et al. The clinical studies of prognostic factors influencing the short-term outcome in patients with acute cerebral infarction. Chongqing Medicine 2014; 43 (5–6): 1075–1077. doi: 10.1159/000460265.
21. Turcato G, Cervellin G, Cappellari M et al. Early function decline after ischemic stroke can be predicted by a nomogram based on age, use of thrombolysis, RDW and NIHSS score at admission. J Thromb Thrombolysis 2017; 43 (3): 394–400. doi: 10.1007/s11239-016-1456-y.
22. Wu Q, Qu J, Yin Y et al. Morning hypertension is a risk factor of macrovascular events following cerebral infarction: a retrospective study. Medicine (Baltimore) 2018; 97 (34): e12013. doi: 10.1097/MD.000000000001 2013.
23. Liu X, Zhang J, Tian C et al. The relationship of leukoaraiosis, haemorrhagic transformation and prognosis at 3 months after intravenous thrombolysis in elderly patients age ≥ 60 years with acute cerebral infarction. Neurol Sci 2020; 41 (11): 3195–3200. doi: 10.1007/s10072-020-04398-2.
24. Lin HC, Chen XY. Effect of regulating serum uric acid level on primary hyperuricemia with mild cognitive impairment. Modern Hospital 2019; 19: 732–733.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2022 Issue 1
Most read in this issue
- Multiple tumefactive brain lesions as the first symptoms of demyelination
- Spontaneous intracranial hypotension
- Deep brain stimulation advances in neurological diseases
- Analytical and pre-analytical aspects of neurofilament light chain determination in biological fluids

