Multiple sclerosis – behind the immunity curtains
Roztroušená skleróza – odhalené tajemství imunity
Roztroušená skleróza je porucha CNS, která u mladých dospělých po celém světě vede k závažné invaliditě a kognitivním poruchám, což má významný emoční a socioekonomický dopad. Je stále více důkazů o tom, že na iniciaci a progresi tohoto onemocnění se podílí mechanizmy související s imunitou. S významným objevem glymfatického systému a meningeálních lymfatických cév, které jsou nezbytné pro odvádění metabolického odpadu z intersticia, došlo v nejnovějších studiích k předefinování jedinečného konceptu chápání mozku jako imunologicky privilegovaného orgánu. V tomto přehledném referátu se stručně věnujeme složitému spojení mezi CNS a periferním imunitním systémem, kdy buňky imunitního systému přestupují hematoencefalickou bariéru a jsou mediátory rozvoje tohoto onemocnění a rovněž mají přístup k zvláštnímu systému lymfatické drenáže mozku s podílem na etiologii RS. Imunitní systém, který má při poškození neuronů a regeneraci tkání kontrastní role, se tak v úsilí o nalezení vhodné léčby stává váženým partnerem.
Klíčová slova:
roztroušená skleróza – vrozená imunita – získaná imunita – glymfatický systém
Authors:
I. S. Barac 1; V. Văcăraș 1; A. Cozma 2; L. M. Procopciuc 3
Authors‘ workplace:
Department of Clinical Neurosciences, „Iuliu Haţieganu“ University of Medicine and Pharmacy, Cluj-Napoca, Romania
1; Department of Internal Medicine, „Iuliu Haţieganu“ University of Medicine and Pharmacy, Cluj-Napoca, Romania
2; Department of Biochimistry, „Iuliu Haţieganu“ University of Medicine and Pharmacy Cluj-Napoca, Romania
3
Published in:
Cesk Slov Neurol N 2020; 83/116(4): 368-374
Category:
Review Article
doi:
https://doi.org/10.14735/amcsnn2020368
Overview
Multiple sclerosis is a CNS disorder that leads to an important disability and cognitive impairment among young adults worldwide, with a meaningful emotional and socioeconomical impact. There is growing evidence pointing to the immune mediated mechanisms involved in the initiation and progression of the disease. Recent studies have redefined the brain’s unique concept of immune privileged organ, with the remarkable discovery of the glymphatic system and the presence of meningeal lymphatic vessels required for the transport of metabolic interstitial waste. In this review, we take a brief look at the complex connection between the central nervous system and the peripheral immune system, in which the immune cells gain access through the brain blood barrier and mediate the disease development and to the brain’s peculiar lymphatic drainage, involved in the etiology of MS. The immune system, with its contrasting roles in neuronal damage and tissue regeneration, becomes a honorable partner in the efforts of finding a well-suited treatment.
Keywords:
Multiple sclerosis – innate immunity – adaptive immunity – glymphatic system
Introduction
Multiple sclerosis is an immune-mediated disorder that targets the CNS, leading to the formation of demyelinated plaques, axonal injuries and astrocytic scars [1]. The inflammatory episodes occurring in the CNS lead to oligodendrocyte injury and death, interrupting the axonal myelin sheet in the optic nerves, brainstem, periventricular white matter, cerebellum and spinal cord [2]. The oligodendrocyte death enhances the production of autoreactive T cells oriented towards the epitope of the myelin oligodendrocyte glycoprotein [2]. The cervical lymph nodes appear to be the main site for autoreactive T cell homing and maturation; therefore, evaluating the connection between the CNS and the peripheral immune system is a matter of current interest [3].
Revolutionary findings in neuroimmunology and MS pathogenicity were possible due to animal models, in which the myelin oligodendrocyte glycoprotein transfer resulted in experimental autoimmune encephalomyelitis (EAE), characterized by the infiltration of perivascular mononuclear cells in the CNS [4].
Multiple sclerosis affects 2.5 million people worldwide, with a female predominance and a high prevalence registered in Northern Europe and Canada [5]. The landmark of the disease is transient neurological deficit lasting more than 24 h, which remits spontaneously, with a relapsing evolution, a hallmark of the relapsing-remitting form (RRMS), consisting of any of the following: dizziness, postural instability, visual loss, sensory disturbances, weakness, spasticity, tiredness or bladder dysfunction [6,7].
Initially, 85% of patients present RRMS with relapses and limited capacity of the CNS to repair the demyelinating plaques, leading to gradual neurological dysfunction and evolving into a secondary progressive form, characterized by cortical atrophy, limited ambulation and cognitive impairment [8,9]. Focal inflammation, demyelinating plaque formation in the white and gray matter, oxidative stress and mitochondrial dysfunction are specific features of RRMS, while secondary progressive multiple sclerosis is associated with axonal loss and neurodegeneration as main causes of progression, inducing permanent disability [8,10]. In other 15% of cases, neurological deficits are progressive from the onset, representing a distinct form of MS known as primary progressive [10].
Multiple sclerosis is considered a multifactorial disease, with a complex interplay between genetic and environmental factors, such as viral infections with Epstein-Barr virus, herpes simplex virus types 1, 2 and 6, varicella-zoster virus, smoking, obesity and a lack of sun exposure and vitamin D deficiency, causing immune dysregulation [7,11].
Regarding genetic risk factors, genome-wide association studies revealed that individuals with MS have an overrepresentation of genes responsible for T helper (Th) cell activation, differentiation, and proliferation [12]. More than 100 genetic variants, known as single nucleotide polymorphisms, were associated with MS risk with most of these genetic loci being related to adaptive immunity [13,14].
Innate immunity
The human immune response to different stimuli develops through an innate component and an adaptive component.
Roles
The innate immune system is a fundamental system of human protection against pathogens, with a central role in modeling the adaptive immune response and removal of antigens, clearance of cellular debris and apoptotic cells, and tissue repair [13].
The main feature of the innate immune system is a quick response, needed for self--protection, but lacking memory and specificity [14]. The innate immune system has the capacity to recognize the molecular patterns expressed on the surface of normal cells, as indicators of regular homeostasis, protecting against an autoimmune response [15]. The CNS innate immune cells have an important role in maintaining an active tolerogenic environment [14].
Cells of the innate immune system
The innate immune system cells are dendritic cells (DCs), mast cells, natural killer (NK) cells, granulocytes, macrophages and microglia in the CNS [13].
Dendritic cells
Dendritic cells are antigen-presenting cells (APCs) derived from myeloid cells activated by the antigen binding on their surface that determine CD4+ T lymphocyte activation, bridging the connection between innate and adaptive immunity [8].
Dendritic cells guide the differentiation process of T cells into effector T cells or regulatory T cells (Tregs), tailoring the adaptive immune response [16].
In the CNS, DCs were described in the choroid plexus and lymphatic vessels of the meninges, exerting their functions as immune sentinels, suggesting that these vessels might be an important route for DC migration from the bloodstream [17].
In MS, DCs present CD83 surface marker expression with an activated phenotype, which might facilitate their migration from the bloodstream through the blood-brain barrier (BBB) [8]. It is noteworthy that in MS, the altered expression of the activation markers on the surface of DCs and their role in controlling Treg differentiation are modified [16].
Mast cells
Mast cells, considered the innate immune system’s first line of defense, are most commonly found in the skin, respiratory tract and gut, as well as in the CNS: choroid plexus, tissue, perivascular space, brain lining and meninges, acting as supervisors of cerebral homeostasis [17,18]. Mast cells contain neuroactive prestored mediators, such as histamine, serotonin, tryptase, chymase, prostaglandin D2 and leukotriene B4, and newly synthesized matrix metalloproteinases, cytokines and chemokines mediating the interaction between neurons, blood vessels and different hematopoietic cells in the CNS [16].
In the CNS, the activated microglia trigger the activation of mast cells that release neuroactive mediators, establishing a sustained inflammatory cycle, opening the BBB and stimulating the recruitment, adhesion and extravasation of leukocytes in the CNS, leading to the destruction of oligodendrocytes and neurons [15,19].
In MS patients, mast cells were detected in the normal-appearing white matter and in demyelinated plaques, with high levels of tryptase and histamine in the cerebrospinal fluid (CSF) [20,21], with an assigned role in the initiation and progression of the disease [17,22].
Natural killer cells
Natural killer cells are bone marrow-derived lymphocytes, functioning as innate immune cells [23]. Their protective role is strengthened by their secretory nature, which classifies them into NK1, secreting interferon g (IFN- g) and interleukin (IL) 10, acting as phagocyte activators, and NK2, secreting IL-5 and IL-13 [24].
The different functional roles divide NK into other distinct types: CD56 dim NK cells, which are highly cytotoxic, and CD56 bright NK, with immunomodulatory functions controlling cytokine production and T cell proliferation and activity [21,25].
In MS, the role of NK is still controversial due to their opposite functions in pathogenesis at different levels. High numbers of immunomodulatory NK are found in the remission phase of MS, suggesting that these cells ameliorate the disease evolution. In contrast to healthy individuals, in MS, immunomodulatory NK have a lower ability to suppress T cell functions; highly cytotoxic NK cells at first remove pathogenic autoreactive T cells and activated microglial cells, but in the process alter the repairing mechanisms [16].
Enhancing the expression of immunomodulatory NK cells in the first stage of the inflammatory process and decreasing the activity of cytotoxic NK cells before altering the regeneration process could be a key to influence MS degeneration.
Granulocytes
Meningeal spaces harbor granulocytes with a primary role in facing pathogen intrusions and tissue damage, with a special enzymatic equipment capable of generating harmful reactive oxygen species (ROS) and reactive nitrogen oxide (NO) species with a strong antimicrobial effect [17,26].
In MS, microglial and mast cells enhance the recruitment of neutrophils in the CNS through the disrupted BBB [19]. In EAE, the number of neutrophils is increased in the peripheral blood and CNS before the onset of symptoms; the inhibition of ROS production demonstrated a clear reduction of clinical severity [27].
The precise contribution of neutrophils in the MS onset and evolution is still under investigation.
Macrophages and microglia
Monocytes are mononuclear cells circulating in the blood, derived from progenitor cells in the bone marrow, which once activated, gain the capacity to pass into tissues and transform into phagocyting macrophages [19].
A microglial cell is a brain specialized type of macrophage that originates from yolk sac macrophages seeding the CNS, early during embryonic development [28].
In the healthy brain, macrophages are harbored in the meninges, perivascular spaces and choroid plexus controlling the brain barriers [29]. Their unique localization at the junction between the brain interstitial fluid and blood might suggest a special role in monitoring CNS activity and homeostasis [17]. There are two different subsets of macrophages: M1, strongly associated with an inflammatory response producing high quantities of IL-1a, IL-6, IL-12, IL-23, tumor necrosis factor a (TNF-a), ROS and NO [18]; M2, with an anti-inflammatory action linked to myelin debris cleaning and oligodendrocyte differentiation during the remyelination process mediated by IL-10, brain-derived neurotrophic factor and insulin-like growth factor-1 [12,22]. In MS, monocytes are constantly recruited from the periphery, becoming macrophages, increasing inflammatory reactions in the CNS, where they act as APCs for T cells, enhancing their traffic through the BBB [19].
Microglia have an important role in brain development, homeostatic and regenerative functions [28]. Microglia as highly specialized cells can induce the acute phase of inflammation, through ROS, NO, IL-1,IL-6, TNF-a and IFN- g production, determining axonal loss and demyelination in a constant oxidative stress state [30,31], contrasting with their capacity to remove the damaged tissues and cellular debris, which favors the resolution of the inflammatory process and remyelination, through the production of growth factors that influence axonal regeneration and oligodendrocyte precursor proliferation [18,19,32].
Macrophages and microglia produce IL-27, a heterodimeric cytokine, involved in inflammation resolution [33].
Switching the functions of microglial cells from neurodestructive to neuroprotective and stimulating their production of IL-27 would be a significant therapeutic achievement, slowing down the evolution of neurodegeneration.
Adaptive immunity
Roles
The constant exposure of the human body to pathogens requires a specific answer known as adaptive immunity, provided by lymphocyte cells that develop into memory cells allowing future responses to already known pathogens [34,35]. The adaptive immune system interactions with different antigens require a large repertoire of antibodies, capable of distinguishing self-antibodies that should be driven away from harmless antigens and alarming ones [36].
Cells of the adaptive immune system
B CELLS
B cells originate in the long bone marrow, being derived from multipotent hematopoietic stem cells [36]. B cell maturation takes place in the peripheral lymphoid organs, leading to the mature form of B cells, plasma cells, which, through antibody production, mediate the humoral immune response [37].
B cells present a unique type of surface receptor (BCR) specialized in antigen recognition, which, throughout its assembly process, can give rise to altered receptors that can interact with the body’s proteins, leading to the development of autoimmune diseases [36]. Two barriers were proposed as controllers for B cell autoreactivity: a central one in the bone marrow, eliminating more than 75% of autoreactive B cells, and a peripheral one, in the secondary lymphoid organs controlled by T regulatory cells [38]. The diversity of our immune system tolerates small amounts of autoreactive cells that in a healthy immune environment will be removed by changing the expression of BCR, or through B cell clonal deletion, or by inactivation in the bone marrow through a process of switching off the B cell receptor to antigen stimulation, a tolerance mechanism known as anergy [36]. Using B cell receptor tracing studies, the cervical lymph nodes were shown to be an important station for B cell traffic between the blood- -stream and CNS [39].
The deep cervical lymph nodes have a major role in helping the immune cells to gain antigen tolerance, host the maturation of B cells before entering the brain and suppressing T cell functions [40]. In MS patients, myelin antigens have been found in the deep cervical lymph nodes [40].
The question that arises is whether there is a way to influence the cervical lymph nodes in order to tailor the disease onset, evolution and severity.
The involvement of B cells in MS immunopathology was demonstrated by the presence of intrathecal synthesized antibodies (in the CSF or within the CNS) in more than 90% of the diagnosed cases, the discovery of meningeal lymphoid aggregates (tertiary lymphoid organs) containing B cells and plasma cells, and by the improvement of the disease evolution and disabilities under specific therapies targeting B cells [16,41]. In MS, B cell autoreactivity is linked to abnormalities of the secondary lymphoid organs [41–43].
T cells
T cells derive from lymphoid hematopoietic stem cells [44]. After thymic development, the maturation process ends when the released T cells arrive to the secondary lymphoid organs including the spleen, lymph nodes and mucosa-associated lymphoid tissue, where they first encounter the antigen and undergo changes in surface phenotype and function [44,45]. Studies showed that in MS, only under specific genetic and environmental influences and with a particular threshold of CNS antigens that reach the deep cervical lymph nodes are T cells able to escape from the thymic controlled self--tolerance mechanism and gain a high avidity for myelin antigens, which, under normal circumstances, can be found within the cervical lymph nodes of healthy individuals without developing autoimmunity [3,30].
The meningeal vessels and choroid plexus were proposed as “security” checks that validate the entrance of immune cells into the CNS and as main routes for their traffic into the tissue [4]. The choroid plexus is unique due to its fenestrated endothelium which may allow a free passage of the immune cells to the CSF, its CSF secreting function, T and B cell hosting [3,46]. Under normal circumstances, between CSF and the blood from the choroid plexus, there is a restrictive barrier, the blood-CSF barrier (BCSFB), which hinders the immune cells from entering the CSF and the brain tissue [3].
In MS, T cells represented by CD4+ T helper cells are first activated in the deep cervical lymph nodes after they encounter APC bearing myelin antigens [30]. Released in the bloodstream as effector cells, they gain a migratory phenotype and extravasate through the choroid plexus and through the pia mater of leptomeningeal vessels, through the modified BBB and BCSFB, into the brain tissue, where they reactivate in the presence of local perivenular APC surrounding periventricular spaces and macrophage cells in the subarachnoid spaces [47], releasing high levels of IFN- g and TNF-a, triggering the occurrence of the first neurological relapse, followed by the activation of resident immune cells and the recruitment of leukocytes from the bloodstream, in a cytokine-dependent manner, resulting in a pro-inflammatory milieu that mediates myelin damage and neuronal dysfunction [3,4,30].
The presence of tertiary lymph nodes in the meninges with germinal centers for B cells and lymphatic vasculature reinforces the assumption that inflammation occurs in the meninges long before the tissue is affected [3,48].
The glymphatic system
Introduction
The concept of “brain as an immune privileged organ” has changed significantly over the past few years. For a long period of time, the brain was considered to be segregated from the peripheral immune system due to the presence of the BBB that restricted the entry of immune cells into the CNS and due to the lack of lymphatic vessels [47]. The classic hypothesis was challenged by extensive studies using special MRI protocols and contrast agents that detected the meningeal lymphatic vessels involved in CSF drainage, opening novel research routes [49].
The theory that the brain, as a unique organ, uses a lymphatic network to eliminate interstitial metabolic products has recently changed. The newly described route, known as glymphatic network, involves glial cells, represented by astrocytes with an important role in mediating metabolic clearance through the presence of distal endfeet aquaporin 4 (AQP4) water channel, and shares common features with the lymphatic system [50,51].
Three different routes contribute to the glymphatic system, in an AQP4-dependent manner: a paraarterial CSF influx route, a transparenchymal route, and a paravenous interstitial fluid clearance route [18].
The glymphatic system is a well organized perivascular network that mimics the activity of a lymphatic pathway in the brain, which allows CSF transport from the subarachnoid space to the periarterial spaces of the large leptomeningeal arteries, the Virchow-Robin spaces, and into the brain tissue in an AQP4-dependent manner, where it blends with the interstitial fluid surrounding the parenchymal cells, moving to the venous perivascular spaces to exit along the perineural sheaths of peripheral nerves and to the meningeal lymphatic vessels to drain into the deep cervical lymph nodes [52–54], as illustrated in Fig. 1.
Obr. 1. Mozkomíšní mok cirkuluje ze subarachnoidálního do periarteriálního prostoru a poté do tkáně přes kanály akvaporinu 4 pro astrocyty, které obklopují mozkové cévy, a při tom se smíchá s intersticiální tekutinou. Intersticiální soluty a buněčné odpadní látky jsou
transportovány z tkáně do perivenózních prostor.
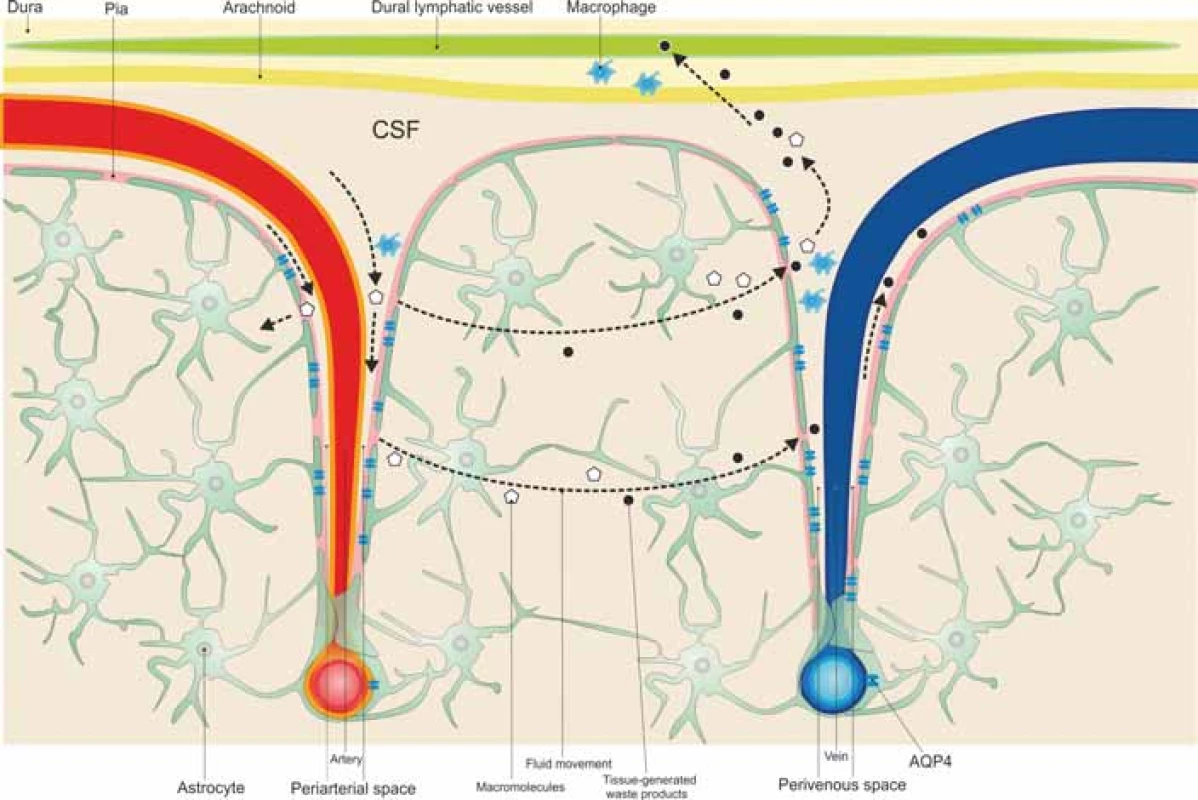
AQP4 – aquaporin 4; CSF – mozkomíšní mok
Lymphatic vessels of CNS
Many efforts have been made to describe the brain’s lymphatic vessels represented by nasal lymphatics, traveling through the dura mater of the cribriform plate, along the olfactory bulb, and dural lymphatics, both draining into the deep cervical lymph nodes [46]. The dural lymphatics have a parallel distribution along the superior sagittal and transverse sinuses, with a similar structure to that of peripheral lymphatic vessels [50].
Lymphatic vessels act as immune sentinels of the CNS, contributors to immune reactions and fluid transporters from the subarachnoid space into the deep cervical lymph nodes and hosts for T lymphocytes, B lymphocytes and dendritic cells [49].
The CSF draining through the nasal lymphatics carries antigen-presenting cells and large molecular weight molecules to the cervical lymph nodes, while the rest of CSF drains through a vacuolar mediated system, from the subarachnoid space to the lymphatic vessels located along the dural sinuses [49,50], as illustrated in Fig. 2.
Obr. 2. Meningeální lymfatické cévy a nosní lymfatické uzliny absorbují intersticiální soluty a buněčné odpadní látky transportované glymfatickým systémem a odvádí je do
mízních uzlin hluboko v krku.
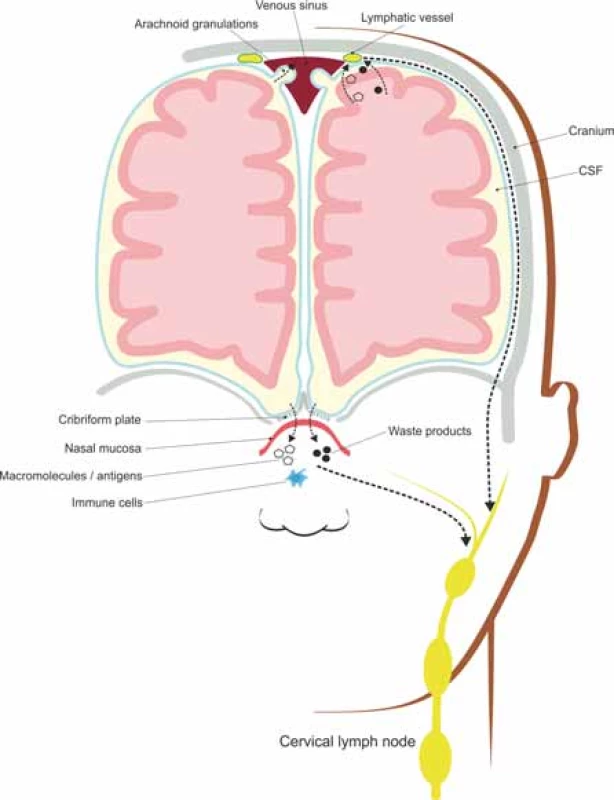
Roles
The glymphatic pathway drains CSF, nutrients and neurotrophic factors in a uniform manner to the entire brain tissue. Experimental mice studies have revealed an enhanced activity of the glymphatic pathway during sleep, eliminating neurotoxic metabolic products from the extracellular space, where they accumulate during wakefulness, and an important role in lactate clearance, in order to stimulate the transition from wakefulness to sleep [53,55].
The glymphatic system in MS
The EAE model of MS revealed that clinical progression is dependent on the influx of immune cells from the perivenous spaces to the tissue [51].
Another hypothesis regarding the succession of pathological events in MS is that a glymphatic pathway dysfunction induces a cerebral perfusion disorder with axonal suffering, which leads to a chronic hypoxic condition, disturbing the energy production by oligodendrocytes and their capacity to produce myelin [56]. The dysfunctional process of myelin formation with high myelin debris, in a chronic hypoxic condition, is a trigger factor for the macrophages harbored in the perivascular spaces and choroid plexus, which associates iron deposition and BBB leakage, increasing myelin and oligodendrocyte damage, which suggests that the inflammatory response represents a reactive, not a primary, response [56]. Another argument for this assumption is a new MRI consensus using the “central vein sign” which splits MS lesions, as a consequence of a restricted outflow, used as a marker in MS differentiation from other neuroinflammatory diseases, suggesting that the battlefield in MS is the perivenular space [57].
Conclusion
Therapeutic success has always been conditioned by the fair understanding of the immunopathological pathways that rise throughout the disease process. In this review, we tried to summarize the various interactions between the immune system and the brain, searching for new directions in understanding MS pathogenesis and therapeutic opportunities. The cerebral lymphatic network description opens new routes for research that may offer a more accurate understanding of the immune cell traffic between periphery and the CNS.
Neuroinflammation is the main factor that draws the line for the subsequent neurodegeneration, influenced at two key points: the perivenular space and the deep cervical lymphatic nodes.
The opposite roles of the cervical lymph nodes, as mediators of immune tolerance versus inducers of autoreactive T cells against myelin antigens, driving neuroimmunological reactions, challenge our imagination to design new therapeutic approaches.
The adequate perfusion of the CNS influenced by the glymphatic pathway may reduce the risk of autoimmune reactions in a healthy nervous environment.
Acknowledgments
The authors would like to thank Claudiu Barac for his kind help and hard work in providing the illustration in our manuscript.
Conflict of interest
The authors declare they have no potential conflicts of interest associated with the manuscript.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Accepted for review: 25. 3. 2020
Accepted for print: 20. 5. 2020
Vitalie Văcăraș, MD
Department of Clinical Neurosciences
„Iuliu Hațieganu“ University of Medicine and Pharmacy
Victor Babes street, nr 43
Cluj-Napoca 400000
Romania
e-mail: vitalievacaras.umf@gmail.com
Sources
1. Kutzelnigg A, Lassmann H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol 2014; 122: 15–58. doi: 10.1016/B978-0-444-52001-2.00002-9.
2. Wang K, Song F, Fernandez-Escobar A et al. The properties of cytokines in multiple sclerosis: pros and cons. Am J Med Sci 2018; 356 (6): 552–560. doi: 10.1016/j.amjms.2018.08.018.
3. Louveau A, Da Mesquita S, Kipnis J. Lymphatics in neurological disorders: A neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron 2016; 91: 957–73. doi: 10.1016/j.neuron.2016.08.027.
4. Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 2016; 353 (6301): 766–771. doi: 10.1126/science.aag2 638.
5. Vasileiadis GK, Dardiotis E, Mavropoulos A et al. Regulatory B and T lymphocytes in multiple sclerosis: friends or foes? Autoimmun Highlights 2018; 9 (1): 9. doi: 10.1007/s13317-018-0109-x.
6. Ponath G, Park C, Pitt D. The role of astrocytes in multiple sclerosis. Front Immunol 2018; 9: 217. doi: 10.3389/fimmu.2018.00217.
7. Ghasemi N, Razavi S, Nikzad E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J 2017; 19 (1): 1–10. doi: 10.22074/cellj.2016.4867.
8. Grigoriadis N, van Pesch V. A basic overview of multiple sclerosis immunopathology. Eur J Neurol 2015; 22 (Suppl 2): 3–13. doi: 10.1111/ene.12798.
9. Sevim S. Relapses in multiple sclerosis: definition, pathophysiology, features, imitators, and treatment. Turkish J Neurol 2016; 22 (3): 99–108. doi: 10.4274/tnd.75318.
10. Salvetti M, Landsman D, Schwarz-Lam P et al. Progressive MS: from pathophysiology to drug discovery. Mult Scler 2015; 21 (11): 1376–1384. doi: 10.1177/ 1352458515603802.
11. Trojano M, Avolio C. Environmental factors and their regulation of immunity in multiple sclerosis. Transl Neuroimmunol Mult Scler From Dis Mech to Clin Appl 2016; 324: 100–111. doi: 10.1016/B978-0-12-801914-6.00008-8.
12. Yadav SK, Mindur JE, Ito K et al. Advances in the immunopathogenesis of multiple sclerosis. Curr Opin Neurol 2015; 28 (3): 206–219. doi: 10.1097/WCO.000000000000 0205.
13. Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 2015; 14 (4): 406–419. doi: 10.1016/S1474-4422 (14) 70305-9.
14. Hossain MJ, Tanasescu R, Gran B. Innate immune regulation of autoimmunity in multiple sclerosis: Focus on the role of Toll-like receptor 2. J Neuroimmunol 2017; 304: 11–20. doi: 10.1016/j.jneuroim.2016.12.004.
15. Fraussen J, de Bock L, Somers V. B cells and antibodies in progressive multiple sclerosis: contribution to neurodegeneration and progression. Autoimmun Rev 2016; 15 (9): 896–899. doi: 10.1016/j.autrev.2016.07. 008.
16. Podbielska M, O’Keeffe J, Hogan EL. Autoimmunity in multiple sclerosis: role of sphingolipids, invariant NKT cells and other immune elements in control of inflammation and neurodegeneration. J Neurol Sci 2018; 385: 198–214. doi: 10.1016/j.jns.2017.12.022.
17. Herz J, Filiano AJ, Smith A et al. Myeloid cells in the central nervous system. Immunity 2017; 46 (6): 943–956. doi: 10.1016/j.immuni.2017.06.007.
18. Perlmutter D. The role of inflammation in neurodegenerative disorders. [online]. Available from URL: https: //www.a4m.com/assets/pdf/bookstore/aamt_vol7_30_perlmutter.pdf.
19. Hernández-Pedro NY, Espinosa-Ramirez G, De La Cruz VP et al. Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin Dev Immunol 2013; 2013: 413465. doi: 10.1155/2013/413465.
20. Stephenson J, Nutma E, van der Valk P et al. Inflammation in CNS neurodegenerative diseases. Immunology 2018; 154 (2): 204–219. doi: 10.1111/imm.12922.
21. McKeon A. Clinical neuroimmunology multiple sclerosis and related disorders. New York: Humana Press 2012.
22. Tuzun E. Immunopathological factors associated with disability in multiple sclerosis. Arch Neuropsychiatry 2018; 55 (Suppl 1): S26–S30. doi: 10.29399/npa.23 303.
23. Durrenberger PF, Ettore A, Kamel F et al. Innate immunity in multiple sclerosis white matter lesions: Expression of natural cytotoxicity triggering receptor 1 (NCR1). J Neuroinflammation 2012: 9: 1. doi: 10.1186/1742-2094-9-1.
24. Gandhi R, Laroni A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol 2010; 221 (1–2): 7–14. doi: 10.1016/j.jneuroim.2009.10.015.
25. Hosseini A, Masjedi A, Baradaran B et al. Dimethyl fumarate: regulatory effects on the immune system in the treatment of multiple sclerosis. J Cell Physiol 2019; 234 (7): 9943–9955. doi: 10.1002/jcp.27930.
26. Sospedra M, Martin R. Immunology of multiple sclerosis. Semin Neurol 2016; 36 (2): 115–127. doi: 10.1055/s-0036-1579739.
27. Woodberry T, Bouffler S, Wilson A et al. The emerging role of neutrophil granulocytes in multiple sclerosis. J Clin Med 2018; 7 (12): 511. doi: 10.3390/jcm7120511.
28. Hänninen A. Infections in MS: An innate immunity perspective. Acta Neurol Scand 2017; 136 (Suppl 201): 10–14. doi: 10.1111/ane.12838.
29. Valente LA, Begg LR, Filiano AJ. Updating neuroimmune targets in central nervous system dysfunction. Trends Pharmacol Sci 2019; 40 (7): 482–494. doi: 10.1016/j.tips.2019.04.013.
30. Naegele M, Martin R. The good and the bad of neuroinflammation in multiple sclerosis. Handb Clin Neurol 2014; 122: 59–87. doi: 10.1016/B978-0-444-52001-2.00 003-0.
31. Adamczyk-Sowa M, Medrek A, Madej P et al. Does the gut microbiota influence immunity and inflammation in multiple sclerosis pathophysiology? J Immunol Res 2017; 2017: 7904821. doi: 10.1155/2017/7904821.
32. Lubetzki C, Stankoff B. Demyelination in multiple sclerosis. Handb Clin Neurol 2014; 122: 89–99. doi: 10.1016/B978-0-444-52001-2.00004-2.
33. Chihara N. Dysregulated T cells in multiple sclerosis. Clin Exp Neuroimmunol 2018; 9 (Suppl 1): 20–29. doi: 10.1111/cen3.12438.
34. Vivier E, Malissen B. Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nat Immunol 2005; 6 (1): 17–21. doi: 10.1038/ni1153.
35. Kim JK, Shin YJ, Ha LJ et al. Unraveling the mechanobiology of the immune system. Adv Healthc Mater 2019; 8 (4): e1801332. doi: 10.1002/adhm.201801332.
36. Merlo LMF, Mandik-Nayak L. Adaptive immunity: B cells and antibodies. 2nd ed. Amsterdam: Elsevier 2013.
37. Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol 2006; 6 (2): 107–116. doi: 10.1038/nri1780.
38. Li R, Patterson KR, Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol 2018; 19 (7): 696–707. doi: 10.1038/s41590-018-0135-x.
39. Palanichamy A, Apeltsin L, Kuo TC et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med 2014; 6 (248): 248ra106. doi: 10.1126/scitranslmed.3008930.
40. Wildner P, Selmaj KW. Multiple sclerosis: skin-induced antigen-specific immune tolerance. J Neuroimmunol 2017; 311: 49–58. doi: 10.1016/j.jneuroim.2017.08.001.
41. Funaro MG, Messina M, Shabbir M et al. The role of B cells in multiple sclerosis: more than antibodies. Discov Med 2016; 22 (122): 251–255.
42. Dhaeze T, Peelen E, Hombrouck A et al. Circulating follicular regulatory T cells are defective in multiple sclerosis. J Immunol 2015; 195 (3): 832–840. doi: 10.4049/jimmunol.1500759.
43. Lazibat I, Majdak MR, Županić S. Multiple sclerosis: new aspects of immunopathogenesis. Acta Clin Croat 2018; 57 (2): 352–361. doi: 10.20471/acc.2018.57.02.17.
44. Luckheeram RV, Zhou R, Verma AD et al. CD4 +T cells: differentiation and functions. Clin Dev Immunol 2012; 2012: 925135. doi: 10.1155/2012/925135.
45. Hendricks DW, Fink PJ. Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage. Blood 2011; 117 (4): 1239–1249. doi: 10.1182/blood-2010-07-299 263.
46. Manglani M, McGavern DB. New advances in CNS immunity against viral infection. Curr Opin Virol 2018; 28: 116–126. doi: 10.1016/j.coviro.2017.12. 003.
47. Engelhardt B, Carare RO, Bechmann I et al. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol 2016; 132 (3): 317–338. doi: 10.1007/s00401-016-1606-5.
48. Lossius A, Johansen JN, Vartdal F et al. High-throughput sequencing of immune repertoires in multiple sclerosis. Ann Clin Transl Neurol 2016; 3 (4): 295–306. doi: 10.1002/acn3.295.
49. Tamura R, Yoshida K, Toda M. Current understanding of lymphatic vessels in the central nervous system. Neurosurg Rev 2020; 43 (4): 1055–1064. doi: 10.1007/s10143-019-01133-0.
50. Al-Kofahi M, Yun JW, Minagar A et al. Anatomy and roles of lymphatics in inflammatory diseases. Anat Roles Lymphat Inflamm Dis 2017; 8 (3): 199–214. doi: 10.1111/cen3.12400.
51. Simon MJ, Iliff JJ. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta 2016; 1862 (3): 442–451. doi: 10.1016/j.bbadis.2015.10.014.
52. Peruzzotti-Jametti L, Pluchino S. Targeting mitochondrial metabolism in neuroinflammation: towards a therapy for progressive multiple sclerosis. Trends Mol Med 2018; 24 (10): 838–855. doi: 10.1016/j.molmed.2018.07.007.
53. Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol Mech Dis 2018; 13: 379–394. doi: 10.1146/annurev-pathol-051217-111018.
54. Weller RO, Carare RO. Lymphatic drainage of the CNS and its role in neuroinflammation and neurodegenerative disease. Amsterdam: Elsevier 2018.
55. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 2018; 17 (11): 1016–1024. doi: 10.1016/S1474-4422 (18) 30318-1.
56. Zamboni P. The Contribution of extra cranial venous drainage to neuro-inflammation in multiple sclerosis. Amsterdam: Elsevier 2018.
57. Sati P, Oh J, Todd Constable R et al. The central vein sign and its clinical evaluation for the diag- nosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 2016; 12 (12): 714–722. doi: 10.1038/nrneurol.2016.166.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2020 Issue 4
Most read in this issue
- It is evident when to make a surgery for lumbar disc herniation?
- CGRP monoclonal antibodies in the treatment of migraine – indication criteria and therapeutic recommendations for the Czech Republic
- Current diagnostics of secondary progressive form of multiple sclerosis and its treatment with siponimod
- Cytotoxic lesions of the corpus callosum (CLOCCs)
