Provocative factors and treatment response in juvenile myoclonic epilepsy – experience from a tertiary epilepsy center
Provokační faktory a reakce na léčbu juvenilní myoklonické epilepsie – zkušenosti z tertiárního epileptického centra
Cíl: Juvenilní myoklonická epilepsie (JME) je epileptický syndrom charakterizovaný myoklonickými záchvaty a dobrou odpovědí na léčbu. Epileptické záchvaty mohou být vyvolány faktory jako spánková deprivace, hlad, stres, blikající jasná světla nebo menstruace. Cílem této studie bylo vyšetřit, zda se provokační faktory v průběhu času mění a zda mají souvislost s odpovědí na léčbu.
Metodika: Do studie bylo zahrnuto 200 pacientů s diagnózou JME, kteří byli sledováni v naší ambulanci. Byly zaznamenávány demografické a klinické charakteristiky pacientů. Přítomnost provokačních faktorů a jejich vývoj v čase byly hodnoceny pomocí osobních rozhovorů s pacienty. Na základě odpovědi na léčbu byli pacienti rozděleni do dvou skupin a porovnáni dle přítomnosti provokačních faktorů a jejich vývoje v čase.
Výsledky: Do studie bylo zařazeno 200 pacientů s JME, jejichž střední věk byl 26,77 ± 8,06 (12–49) let. Alespoň jeden provokační faktor byl přítomen u 199 pacientů (99,5 %). Nejčastějšími provokačními faktory byla spánková deprivace (166 pacientů, 83 %), stres (151 pacientů, 75,5 %) a únava (125 pacientů, 62,5 %). Odpověď na léčbu byla méně uspokojivá u pacientů s perzistentní citlivostí na spánkovou deprivaci, stres, únavu, hlad, světelnou stimulaci a smutek (p < 0,05).
Závěr: Provokační faktory se v průběhu času mohou vyvíjet, ale perzistentní přítomnost provokačních faktorů epileptického záchvatu může u pacientů s JME naznačovat, že odpověď na léčbu bude méně uspokojivá. Tyto výsledky ukazují, že věnování pozornosti provokačním faktorům pomáhá nejen při prevenci epileptických záchvatů, ale také při předpovědi odpovědi na léčbu.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Klíčová slova:
epilepsie – provokační faktory – juvenilní myoklonická epilepsie – epileptický záchvat – léčba
Authors:
K. Mulhan; B. Tekin; M. Erdoğan; H. Sari; M. D. Daryan; D. Atakli
Authors‘ workplace:
Mazhar Osman Mental Health and Neurological Diseases Education and Research Hospital, Istanbul, Turkey
; Department of Neurology, Bakirköy
Published in:
Cesk Slov Neurol N 2020; 83(2): 180-183
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn2020180
Overview
Aim: Juvenile myoclonic epilepsy (JME) is an epilepsy syndrome characterized by myoclonic seizures and good response to treatment. Factors such as sleep deprivation, hunger, stress, bright flashing lights and menstruation may provoke seizures. The objective of this study was to investigate these provocative factors, whether they display changes over time and have a relation to treatment response.
Methods: 200 patients with a JME diagnosis who are being followed in our outpatient clinic were included in the study. The demographic and clinical characteristics of the patients were recorded. The provocative factor presence and temporal evolution of these factors were investigated in face-to-face interviews with patients. The patients were categorized into two groups based on treatment response and compared according to the presence and temporal evolution of the provocative factors.
Results: 200 JME patients were enrolled and the mean age was 26.77 ± 8.06 (12–49) years. At least one provocative factor was identified in 199 patients (99.5%). The most common provocative factors were sleep deprivation, in 166 (83%), stress in 151 (75.5%) and fatigue in 125 (62.5%) of patients. The response to treatment was less satisfactory in patients with persistent sensitivity to sleep deprivation, stress, fatigue, hunger, photo stimulation and sadness (P < 0.05).
Conclusion: Provocative factors may evolve over time, but the persistent presence of seizure-provocative factors in patients with JME may indicate that the treatment response will be less satisfactory. These results show that paying attention to provocative factors is not only helpful for providing means to prevent seizures, but also for predicting the treatment response.
Keywords:
Epilepsy – treatment – provocative factors – juvenile myoclonic epilepsy – seizure
Background
Juvenile myoclonic epilepsy (JME) is a primary generalized epilepsy syndrome classified amongst genetic generalized epilepsies. It is characterized by myoclonic jerks, generalized tonic-clonic seizures and absence seizures. Patients with JME are particularly susceptible to seizure facilitation as a result of sleep deprivation, stress, consumption of alcohol or flashing lights and playing video games [1–3]. Under appropriate antiepileptic drug (AED) treatment, up to 88% of patients become seizure-free, but they may relapse after AED withdrawal [4–6]. It has been generally accepted that JME is a lifelong disorder and it is unwise to discontinue treatment once seizure control has been established. This pessimistic outlook has been challenged in recent population-based studies [4–9]. It seems that some patients remain seizure-free and in some cases, do not even require treatment. Our aim was to investigate the changes in seizure-provoking factors and their relationship with treatment response in JME patients.
Methods
We included patients who had been diagnosed with JME according to the classification of the International League Against Epilepsy (2017) in epilepsy outpatient clinic at the Department of Neurology, Bakirköy Mazhar Osman Mental Health and Neurological Diseases Education and Research Hospital, Istanbul, Turkey [1]. The patient data were analyzed based on medical records and face-to-face interviews. The sociodemographic data (age, gender, education) and clinical characteristics of the patient (family history of epilepsy, consanguinity of parents, history of febrile convulsions, duration of disease, seizure frequency as well as details about the medication used) were reviewed in the medical records. Each patient was asked to answer a structured questionnaire (modified from literature [1–3] by us) about the provocative factors and their temporal evolution.
If the provocative factor had caused seizures at a given time in the patient’s lifetime, but now has no effect on seizures, it is defined as a “temporary provocative factor”.
To determine the changes in provocative factors in years, patients with at least 10 years of epilepsy who were older than 30 years of age were evaluated. Then, the patients were divided into two groups based on seizure control as treatment-responsive and treatment-resistant. The treatment-resistant group included patients who had more than two myoclonic seizures in a month or more than one generalized tonic clonic seizure in a year. The temporal variability of provocative factors was compared between treatment-responsive and treatment-resistant patients.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation; categorical variables are presented as frequency and percentage. The chi-square test was used to compare the differences in categorical variables between the groups. SPSS 17.0 statistical software (IBM, Armon, NY, USA) was used for statistical analysis. A P < 0.05 was considered statistically significant.
Results
We identified 200 patients (112 female), the mean age was 26.77 ± 8.06 (12–49) years. Their demographic data are shown in Tab. 1. In 199 (99.5%) patients, at least one provocative factor was detected and the mean number of provocative factors was 4.92 ± 3 (1–16). Only one patient reported no provocative factors. The most common provocative factors were sleep deprivation 166 (83%), stress 151 (75.5%) and fatigue 125 (62.5%) (Tab. 1).
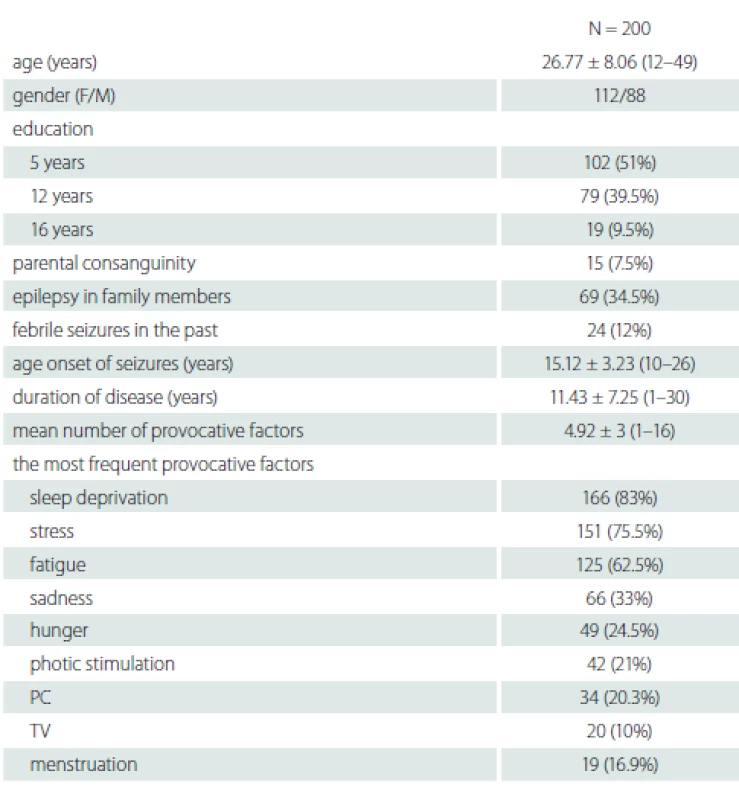
Fifty-four patients over the age of 30 with more than 10 years of disease history selected to determine the changes of the provocative factors over time are shown in Tab. 2. The mean disease duration of patients who were older than 30 years of age was 19.89 ± 5.2 (10–30) years. There was no statistically significant relationship between the variability of provocative factors and age, gender, education, parental consanguinity and family history of epilepsy (P > 0.05).
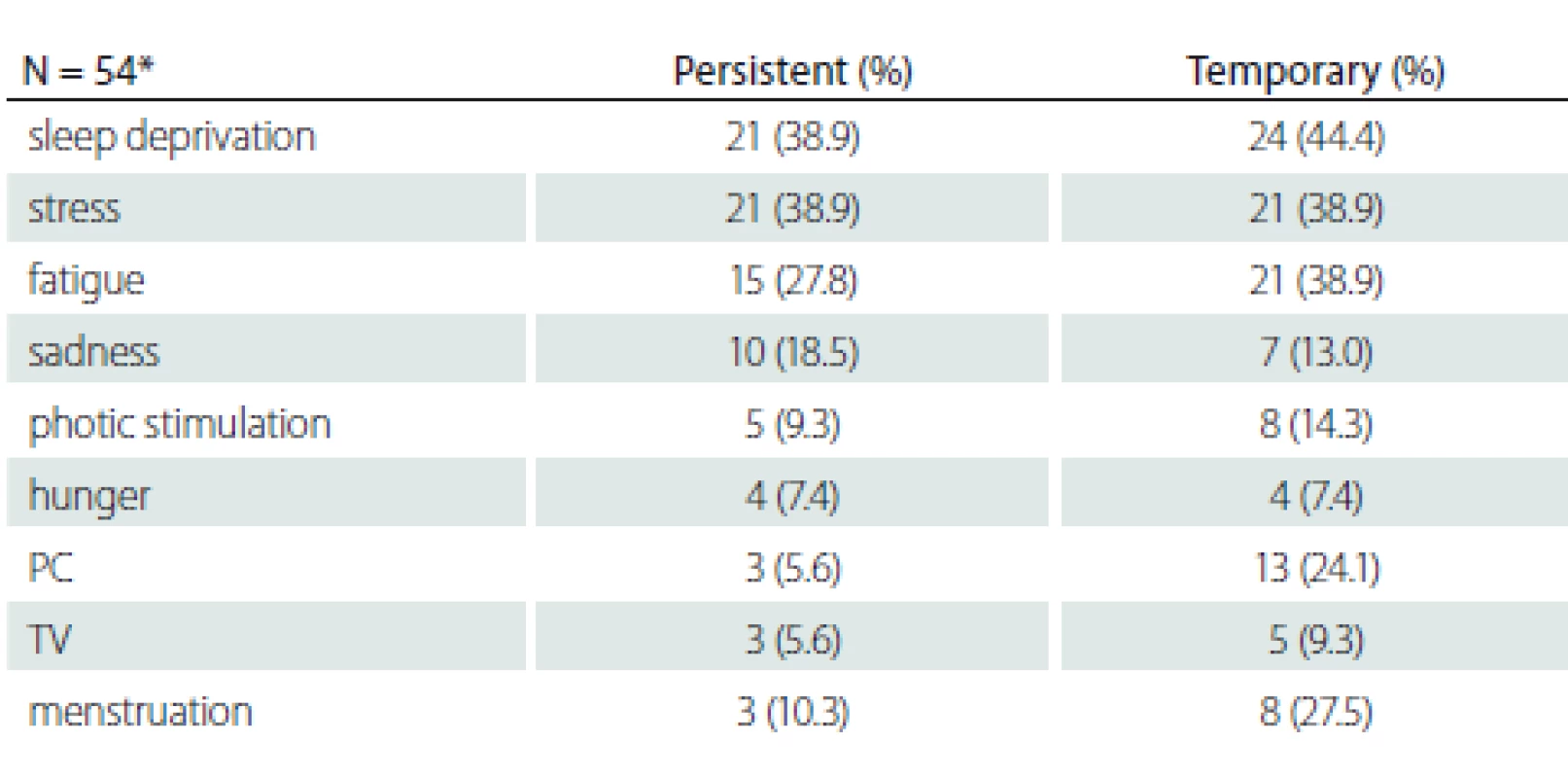
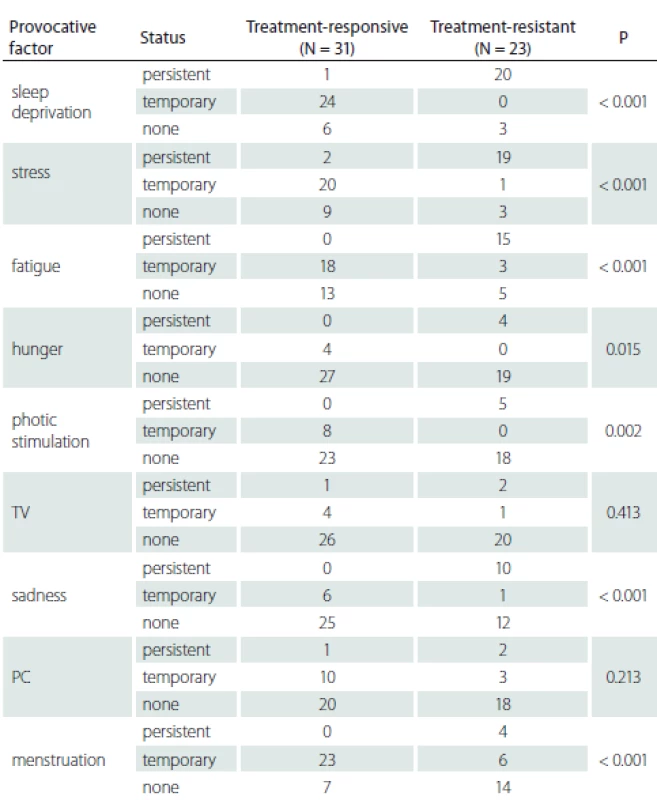
Patients with persistent provocative factors such as sleep deprivation, stress, fatigue, hunger, photo stimulation and sadness responded less to treatment and this difference was statistically significant (P < 0.05). It was found that patients whose seizures were temporarily triggered by menstruation had a better prognosis and that they responded to treatment better than patients whose seizures were never triggered or always triggered by menstruation (P < 0.001) (Tab. 3).
Discussion
Although seizures in epilepsy occur spontaneously, it is also known that there are endogenous and exogenous factors that trigger these seizures [10,11]. Janz and Christian defined the provocative factors in JME for the first time in 1957 and pointed out sleep deprivation, stress, fatigue, brightly lit environments, menstruation and excessive use of alcohol as the most common provocative factors [12].
Up to 90% of patients with JME report that their seizures are provoked by a variety of general factors, such as stress, fatigue, fever and sleep. In our study, this number was found to be slightly higher. The reason for this could be that our survey was designed to include a number of details. The most common provocative factors were found to be stress, sleep deprivation and fatigue, though the rates varied in particular studies [13–15]. The results in our study support those findings in literature. It was noteworthy that hunger and sadness were among the common provocative factors in addition to other emphasized factors in our study. Hunger had not previously attracted attention as a provocative factor. We believe that hunger is an important factor because it comes to light especially during Ramadan. Ramadan is a month of the lunar calendar. People fast from sunset to sundown during Ramadan and any type of drinking or eating is prohibited. In years when Ramadan takes place in the summer according to the Gregorian calendar, fasting may last up to 16 h in Turkey. Patients usually take their medicine before sunset or after sundown with meals. Controlled-release pills have efficacy over the day so we believe there may be another factor for the seizures. Hunger may be a better explanation for this phenomenon. In terms of response to treatment and prognosis in JME, Penry et al suggest that JME is a disease that requires life-long treatment [16]. Several long-term observational studies of JME patients with a follow-up of at least 20 years have been published since the year 2000. In 2006, a study from Los Angeles found that in 9% of patients, JME might not be life-long [17]. Baykan et al showed a substantial alleviation of myoclonic seizures in the fourth decade of life. They had 48 patients who were followed up for a mean of 20 years, five of them had stopped AED treatment and six were on a lower dose of AED in comparison with the dosage needed to control the seizures at the beginning [4]. Camfield et al suggest that in about one-third of patients with JME, troublesome seizures vanish and eventually, daily AED treatment is no longer required. All seizure types remit in about 17% and in 13%, only myoclonic seizures persist for up to 22 years after stopping AED [5]. In the Greifswald series (2012) the outcome in 31 patients with JME after a median follow-up of 39 years was evaluated. 67.7% became seizure-free on AEDs and AED treatment was discontinued in 28.6% of patients [7]. In another study, after a mean follow-up period of 45 years, 59% out of 66 patients remained free of seizures for at least 5 years prior to the last follow-up. 71.8% of seizure-free patients were still taking AEDs and only 28.2% were off AEDs for at least the last 5 years of the follow-up [9]. Höfler et al showed that 9% of JME patients had been seizure-free for more than 2 years without AED treatment [8]. New studies focusing on provocative factors and their relationships with prognosis and response to therapy in JME are needed.
It has come to our attention that provocative factors that represent an important clinical feature of JME, were not discussed in previous studies related to clinical prognosis and response to therapy. Camfield and Camfield suggested that it was possible that the relatively optimistic rate of remission in their study might be related to the fact that seizure-provoking factors such as binge drinking and sleep deprivation were likely to decrease with age [5]. We investigated how much of an effect provocative factors have on JME prognosis.
Our study showed that in some of our patients, seizure-inducing factors occured only in a certain period of patient’s life and then disappear; in some of our patients, these factors affected their lives much longer.
We found that response to therapy was lower in the group of patients with persisting provocative factors, such as stress, fatigue and sleep deprivation. It is reasonable to think that some of patients pay attention to these provocative factors and change their lifestyles accordingly. Naturally, the prognosis of these patients is better. This responsive group also stated clearly that these described provocative factors did not provoke seizures as previously. Furthermore, although sleep deprivation is a factor that can be avoided, stress and fatigue are hard to avoid in our daily lives. It is also noteworthy that menstruation provoked seizures in some patients during a given time period. These findings give us the idea that in at least one group of patients, the provoking factors lose their provoking effects over time and that this group responds better to treatment. Patients whose treatments could be stopped in the previous studies and patients who state that the provocative factors lose their effect to trigger seizures over time, may be counted in the same group. We may be neglecting the investigation of provocative factors and focusing on seizure frequency in daily practice. However, provocative factors occur more often than previously thought and have a dynamic nature in themselves. Provocative factors may be used in predicting treatment response as well as giving patients information to prevent them from having seizures. We believe that a short form prepared for patients in outpatient settings may be useful both for informing patients and for follow-up. The reason we have not found a significant correlation between other factors and treatment response may be explained by the low numbers of those factors. Should the study be repeated with more patients, these factors may show significance.
We can say that JME patients are a heterogeneous group and that in some of these patients, provocative factors continue to have provocative effect on seizures for a long time, maybe even for life, and that their treatment response is worse.
Conclusion
Juvenile myoclonic epilepsy is known to be a heterogeneous disease group both in clinical characteristics and treatment response. Provocative factors may have an influence on treatment response, and this could persist for years or evolve over time. This is important not only for giving advice to the patients to prevent seizures, but it may also be useful for predicting treatment response.
Ethical principles
This study was conducted under the permission of the Ethics Committee of Bakirkoy Education and Research Hospital for Psychiatry, Neurology, and Neurosurgery (number 2014-70787). The details of the study were explained to the patients and written informed consent forms were taken.
Disclosures
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Assoc. Prof. Betül Tekin, MD
Department of Neurology
Bakirköy Mazhar Osman Mental
Health and Neurological Diseases
Education and Research Hospital
Zuhuratbaba-Bakırkoy 34147
Istanbul
Turkey
e-mail: betultekin2013@gmail.com
for review: 7. 5. 2019
Accepted for print: 18. 12. 2019
Sources
1. Scheffer IE, Berkovic S, Capovilla G et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017; 58 (4): 512–521. doi: 10.1111/epi.13709.
2. Patsalos PN, Froscher W. The importance of drug interactions in epilepsy therapy. Epilepsia 2002; 43 (4): 365–385. doi: 10.1046/j.1528-1157.2002.13001.x.
3. Thomas P, Genton P, Gelisse P et al. Epileptic syndromes in infancy, childhood and adolescence. 3rd ed. Barnet, UK: John Libbey 2002: 335–355.
4. Baykan B, Martínez-Juárez IE, Altindag EA et al. Lifetime prognosis of juvenile myoclonic epilepsy. Epilepsy Behav 2013; 28 (Suppl 1): S18–S24. doi: 10.1016/j.yebeh.2012.06.036.
5. Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: a population-based study. Neurology 2009; 73 (13): 1041–1045. doi: 10.1212/WNL.0b013e3181b9c86f.
6. Syvertsen MR, Thuve S, Stordrange BS et al. Clinical heterogeneity of juvenile myoclonic epilepsy: Follow-up after an interval of more than 2 years. Seizure 2014; 23 (5): 344–348. doi: 10.1016/j.seizure.2014.01.012.
7. Geithner J, Schneider F, Wang Z et al. Predictors for long-term seizure outcome in juvenile myoclonic epilepsy: 25–63 years of follow-up. Epilepsia 2012; 53 (8): 1379–1386. doi: 10.1111/j.1528-1167.2012.03526.x.
8. Höfler J, Unterberger I, Dobesberger J et al. Seizure outcome in 175 patients with juvenile myoclonic epilepsy – a long-term observational study. Epilepsy Res 2014; 108 (10): 1817–1824. doi: 10.1016/j.eplepsyres.2014.09. 008.
9. Senf P, Schmitz B, Holtkamp M et al. Prognosis of juvenile myoclonic epilepsy 45 years after onset: seizure outcome and predictors. Neurology 2013; 81 (24): 2128–2133. doi: 10.1212/01.wnl.0000437303.36064.f8.
10. Aird RB, Gordon NS. Some excitatory and inhibitory factors involved in the epileptic state. Brain Dev 1993; 15 (4): 299–304. doi: 10.1016/0387-7604 (93) 90028-7.
11. Janz D, Christian W. Impulsive petit mal. Deutsche Zeitschrift Nervenheilkunde 1957; 176 (3): 346–386.
12. Montalenti E, Imperiale D, Rovera A et al. Clinical features, EEG findings and diagnostic pitfalls in juvenile myoclonic epilepsy: a series of 63 patients. J Neurol Sci 2001; 184 (1): 65–70. doi: 10.1016/s0022-510x (00) 00496-2.
13. da Silva Sousa P, Lin K, Garzon E et al. Self-perception of factors that precipitate or inhibit seizures in juvenile myoclonic epilepsy. Seizure 2005; 14 (5): 340–346. doi: 10.1016/j.seizure.2005.04.007.
14. Shahnaz, Sher K, Abdul Sattar R. Clinical and EEG characteristics of juvenile myoclonic epilepsy. Pak J Med Sci 2014; 30 (1): 12–15. doi: 10.12669/pjms.301.4465.
15. Kasteleijn-Nolst Trenité DG, de Weerd A, Beniczky S. Chronodependency and provocative factors in juvenile myoclonic epilepsy. Epilepsy Behav 2013; 28 (Suppl 1): S25–S29. doi: 10.1016/j.yebeh.2012.11.045.
16. Penry JK, Dean JC, Riela AR. Juvenile myoclonic epilepsy: long-term response to therapy. Epilepsia 1989; 30 (Suppl 4): S19–S23. doi: 10.1111/j.1528-1157.1989.tb05833.x.
17. Martínez-Juárez IE, Alonso ME, Medina MT et al. Juvenile myoclonic epilepsy subsyndromes: family studies and long-term follow-up. Brain 2006; 129 (Pt 5): 1269–1280. doi: 10.1093/brain/awl048.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2020 Issue 2
Most read in this issue
- Cavum septi pellucidi, cavum vergae and cavum veli interpositi
- Vascular morphology, symptoms, diagnostics and treatment of brainstem ischemic stroke
- Surgical treatment of brain metastases
- The International Classification of Headache Disorders (ICHD-3) – the official Czech translation
