Malignant melanotic schwannoma of the vertebral body in a patient with Carney complex
Maligní melanotický schwannom obratlového těla u pacienta s Carneyho komplexem
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
T. S. Jeong; S. G. Lee; W. K. Kim; N. R. Kim
Authors‘ workplace:
Department of Neurosurgery, Gachon University Gil Medical Center, Incheon, Republic of Korea
Published in:
Cesk Slov Neurol N 2018; 81(2): 226-228
Category:
Letter to Editor
doi:
https://doi.org/10.14735/amcsnn2018226
Overview
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Dear Editor,
Schwannomas are benign tumours originating from the Schwann cells that constitute the nerve sheath [1]. Melanotic schwannoma is a rare variant that is capable of melanogenesis [2]. Most melanotic schwannomas are benign, but 10% are malignant with metastasis [3]. We report a case of malignant spinal melanotic schwannoma found in the vertebral body and nerve root.
A 33-year-old man suffering from back and neck pain for 6 months was admitted because of aggravation of the neck pain restricting his movement over the previous 3 days. He underwent excision of cardiac myxoma in the right ventricle and atrium 6 years previously, and bilateral adrenalectomy because of familial Cushing syndrome 2 years ago. Neurologically, motor power was intact, but he complained of severe neck and back pain. The pain pattern of the neck was a severe axial pain without irradiation, and the back pain was severe tenderness in the middle part of the back.
Preoperative spine MRI showed osteolytic bone lesions of the vertebral body, right pedicle, and articular process of C5, the vertebral body of T12 and L1, and the L1 left pedicle with enhancment on T1-weighted image with gadolinium administration, and showed a dumbbell-shaped mass lesion in the right lateral recess and foramen at the C3– C4 level (Fig. 1). Preoperative positron emission tomography/ computed tomography (PET/ CT) was performed. Pathologic lesions were observed in C3, C5, T12, L1, and the lateral arc of the left 4th rib. Except for that, abnormal hypermetabolic lesions suspected of malignancy were not observed.
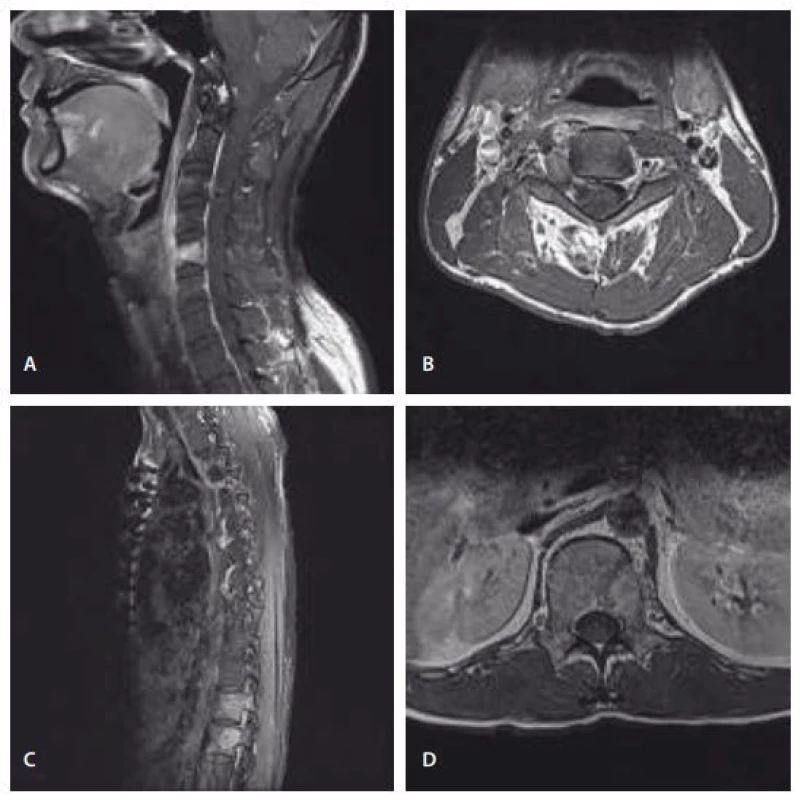
The patient underwent a bone biopsy of the left L1 pedicle, and histopathological examination revealed epithelioid cells with marked brown pigment. The first surgery was performed 2 months after the biopsy. C5 body was resected piecemealy using a cavitron ultrasonic aspirator (CUSA), but transverse foramen, articular facets, pedicles, laminas, and spinous process were not removed. Interbody fusion and fixation were performed using titanium mesh cage and anterior cervical plate. Surgical findings revealed that a soft black mass with high vascularity had invaded into the C5 body and there was no invasion of the tumour or bone displacement into the epidural space. A second surgery for the thoracolumbar lesion was performed 10 days after the first one. In the right lateral decubitus position, subtotal removal of the T12 body and partial removal of the L1 body were achieved using a CUSA by the transthoracic approach. Interbody fusion was performed using an expandable cage, and the posterolateral screws were fixed to Th10, 11, L2, 3. The T12 and L1 vertebral bodies were invaded by a soft black mass like that affecting the C5 body, and there was an invasion of the tumour into the epidural space behind the T12 body. C4 nerve root lesion was planned to be removed, but the general condition of the patient was suddenly worse 1 month after the second operation, and the operation could not proceed.
One month after the second surgery, PET/ CT and chest CT were performed. PET/ CT showed bone metastases with new hypermetabolism in C2, T11, L3, right humerus, femur, 8th rib and pulmonary metastasis with multiple hypermetabolic nodules in both lungs, and chest CT showed pleural effusion and small pulmonary nodules in both lung fields. A core needle lung biopsy was carried out. As a result, lung metastasis was diagnosed. The patient was subsequently treated with radiotherapy and combination chemotherapy with doxorubicin and dacarbazine. However, he died eight months after the second surgery because of worsening of respiratory condition.
The surgical specimen consisted of aggregates of the pigmented fragmented tumour. Histologically, there were darkly pigmented ovoid tumour cells, and the mass had invaded the vertebrae (Fig. 2A, B). The nuclei were round and vesicular with occasional prominent nucleoli (Fig. 2C). Psammoma bodies were not found. The cell contour was obscured by dark black melanin pigments. A lung biopsy also showed similar findings (Fig. 2D). On immunohistochemical examination, the tumour cells showed intense cytoplasmic and nuclear expression of S-100 protein (polyclonal antibody, 1:600 dilution, Dako, Denmark) and human melanoma black 45 (HMB45, prediluted, Dako). A molecular study for the BRAF gene V600E mutation and sequencing was performed, and a BRAF mutation was not detected. The patient was diagnosed with malignant melanotic schwannoma.
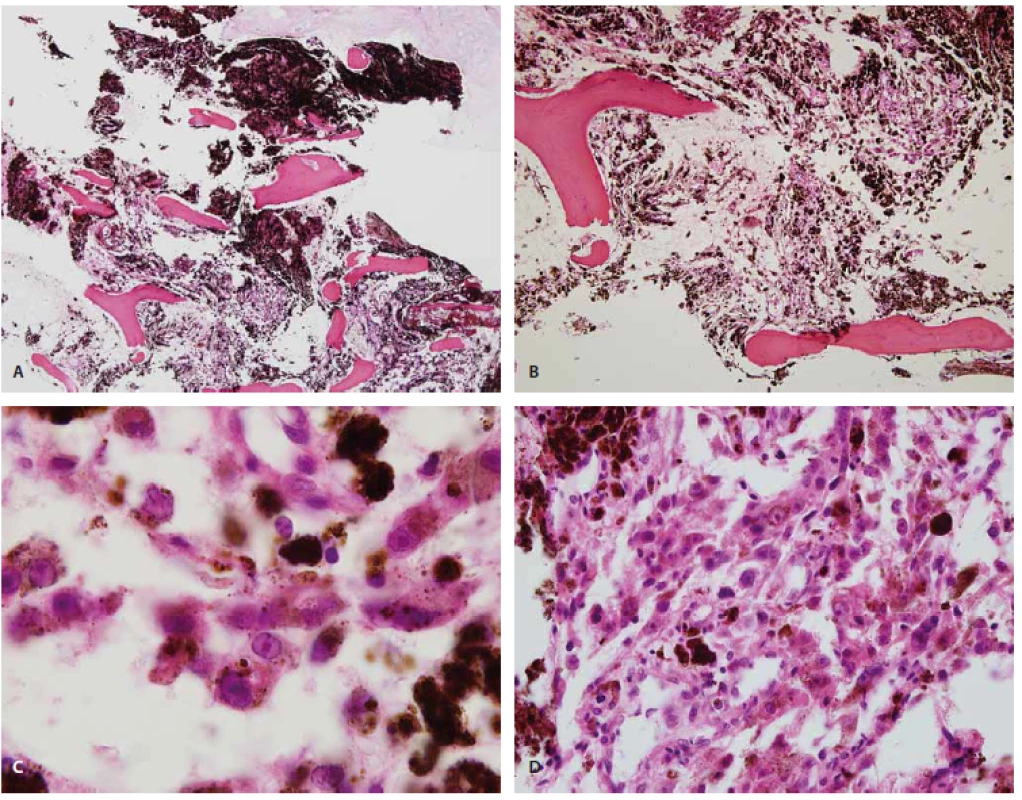
Melanotic schwannoma has light and electron-microscopic features in common with melanoma; these include high cellularity and cell pleomorphism with prominent nucleoli, usually without mitosis [2]. In 1990, Carney [4] studied 31 patients with melanotic schwannomas. In 55% of these patients, clinical evidence of the Carney complex, consisting of myxomas (heart, skin, and breast), spotty pigmentation (lentigines and blue nevi), and endocrine overactivity (Cushing syndrome or acromegaly) was shown.
Peripheral nerve sheath tumours (PNSTs) are a group of primary neurogenic tumours that arise from nerve sheaths outside of the CNS. Malignant schwannoma belongs to the group of PNSTs [5]. Although descriptions of the course of melanotic schwannoma differ somewhat [6,7], the lesions are generally benign and are rarely malignant [4,8]. Melanotic schwannoma arising from the vertebral body is extremely rare, and few of these cases develop metastases [2]. In our case, pathological analysis of the biopsies obtained at vertebral bodies revealed malignant melanotic schwannoma, which was a distinctive malignant neoplasm rather than a simple schwannoma variant. One month after surgery, lung metastases were confirmed. When comparing pre- and postoperative PET/ CT, metastases progressed to various sites, such as the lung, humerus, femur, and ribs. The nature of the tumour seems to have a very rapid metastases. However, it is also possible that the metastases may have progressed rapidly due to surgery by the transthoracic approach. Because some studies have reported the effectiveness of radiation and various chemotherapeutic agents [6,8], radiotherapy and combination chemotherapy were performed. However, the effect could not be confirmed because the patient died.
Melanotic schwannomas are usually benign, but the malignant type tends to metastasize. Carney complex is associated with malignant melanotic schwannomas. Patients diagnosed with Carney complex should be evaluated for various characteristics of this syndrome, including spinal tumours, and be followed closely for the possibility of metastases or recurrence.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 9. 6. 2017
Accepted for print: 19. 2. 2018
Sang Gu Lee MD, PhD.
Gachon University Gil Medical Center
21 Namdong-daero 774 beon-gil
Namdong-gu, Incheon 405760
Republic of Korea
e-mail: samddal@gilhospital.com
Sources
1. De Cerchio L, Contratti F, Fraioli MF. Dorsal dumb-bell melanotic schwannoma operated on by posterior and anterior approach: case report and a review of the literature. Eur Spine J 2006; 15 (Suppl 5): 664– 669. doi: 10.1007/ s00586-006-0205-x.
2. Vallat-Decouvelaere AV, Wassef M, Lot G et al. Spinal melanotic schwannoma: a tumour with poor prognosis. Histopathology 1999; 35(6): 558– 566.
3. Shields LB, Glassman SD, Raque GH et al. Malignant psammomatous melanotic schwannoma of the spine: a component of Carney complex. Surg Neurol Int 2011; 2: 136. doi: 10.4103/ 2152-7806.85609.
4. Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol 1990; 14(3): 206– 222.
5. Panigrahi S, Mishra SS, Das S et al. Primary malignant peripheral nerve sheath tumor at unusual location. J Neurosci Rural Pract 2013; 4 (Suppl 1): S83– S86. doi: 10.4103/ 0976-3147.116480.
6. Killeen RM, Davy CL, Bauserman SC. Melanocytic schwannoma. Cancer 1988; 62(1): 174– 183.
7. Mennemeyer RP, Hallman KO, Hammar SP et al. Melanotic schwannoma. Clinical and ultrastructural studies of three cases with evidence of intracellular melanin synthesis. Am J Surg Pathol 1979; 3(1): 3– 10.
8. Lowman RM, Livolsi VA. Pigmented (melanotic) schwannomas of the spinal canal. Cancer 1980; 46(2): 391– 397.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2018 Issue 2
Most read in this issue
- Ataxia
- Brain biopsy in 10 key points – what can a neurologist expect from the neurosurgeon and the neuropathologist?
- Fabry disease, an overview and the most common neurological manifestations
- Glucose transporter-1 deficiency syndrome – expanding the clinical spectrum of a treatable disorder
