Hladiny neurotrofického faktoru odvozeného od gliových buněk a nervového růstového faktoru v séru u pacientů s onemocněním COVID-19
Hladiny neurotrofického faktoru odvozeného od gliových buněk a nervového růstového faktoru v séru u pacientů s onemocněním COVID-19
Úvod a cíl: Nový typ koronaviru (COVID-19) způsobuje vysokou horečku, únavu, kašel, dechové potíže, průjmy, u některých pacientů bolesti hlavy, cerebrovaskulární onemocnění, bezvědomí, encefalopatii, encefalitidu, poškození periferního nervového systému atd. Jedná se o virové respirační onemocnění, které se projevuje neurologickými nálezy. V naší studii byly zkoumány hladiny neurotrofického faktoru odvozeného od glií (glial-derived neurotrophic factor; GDNF) a nervového růstového faktoru (nerve growth factor; NGF) neurotrofických faktorů (NF), které zajišťují přežití, růst, zrání a diferenciaci neuronů u pacientů s COVID-19 a jejich vztah se závažností onemocnění. Materiál a metody: Z celkového počtu 70 účastníků je 20 účastníků ve zdravé kontrolní skupině (CG) a 50 účastníků je ve skupině pacientů s COVID-19 dle PCR testu (nekomplikovaná skupina [NCG], středně těžká skupina [MG], těžká skupina [SG]). Hladiny NGF a GDNF v séru ve všech skupinách byly hodnoceny spektrofotometricky pomocí metody ELISA. Výsledky byly porovnány jak mezi skupinami pacientů, tak mezi pacienty a kontrolní (zdravou) skupinou. Výsledky: Koncentrace NGF v séru byla signifikantně vyšší ve skupině MG než ve skupinách NCG a SG (p = 0,042). U sérových hladin GDNF u pacientů s COVID-19 a CG nebyly zjištěny statisticky významné rozdíly. Závěr: Nebyl zjištěn žádný rozdíl v sérových hladinách NGF a sérových hladinách GDNF u pacientů s onemocněním COVID-19 ve srovnání se zdravou kontrolní skupinou.
Klíčová slova:
COVID-19 – koronavirus – neurotrofický faktor odvozený od gliových buněk – nervový růstový faktor
Authors:
O. N. Turkeri 1; F. B. Ozgeris 2; Ö. F. Kocak 3; N. Kurt 4; N. Yuce 5; N. Bakan 6; E. Parlak 7
Authors place of work:
Department of Pharmacy Services, Çanakkale Health Services Vocational School, Çanakkale Onsekiz Mart University, Çanakkale, Turkey
1; Department of Nutrition and Dietetics, Faculty of Health Sciences, Atatürk University, Erzurum, Turkey
2; Department of Chemistry and Chemical Process Technologies, Erzurum Vocational College, Atatürk University, Erzurum, Turkey
3; Medical Biochemistry, Faculty of Medicine, Erzincan Binali Yildirim University, Erzincan, Turkey
4; Department of Medical Biochemistry, Faculty of Medicine, Atatürk University, Erzurum, Turkey
5; Medical Biochemistry, Faculty of Medicine, Atatürk University, Erzurum, Turkey
6; Department of Infection Diseases, Faculty of Medicine, Atatürk University, Erzurum, Turkey
7
Published in the journal:
Cesk Slov Neurol N 2023; 86(2): 128-133
Category:
Původní práce
doi:
https://doi.org/10.48095/cccsnn2022128
Summary
Introduction and objective: The new type of coronavirus (COVID-19) causes high fever, fatigue, cough, respiratory distress, diarrhea, headache in some patients, cerebrovascular diseases, unconsciousness, encephalopathy, encephalitis, peripheral nervous system damage, etc. It is a viral respiratory disease that manifests itself with neurological findings. In our study, glial-derived neurotrophic factor (GDNF) and nerve growth factor (NGF) levels of neurotrophic factors (NF), which ensure the survival, growth, maturation and differentiation of neurons were investigated in COVID-19 patients, including their relationship with the severity of the disease. Materials and methods: Out of a total of 70 participants, 20 participants are in the healthy control group (CG) and 50 participants are in the group of patients with COVID-19 according to PCR test (uncomplicated group [NCG], moderately severe group [MG], severe group [SG]). Serum NGF and GDNF levels in all groups were evaluated spectrophotometrically using ELISA kits. The results were compared both between the patient groups and between the patient and healthy control groups. Results: Serum NGF concentration was significantly higher in the MG group than in the NCG and the SG group (P = 0.042). No statistically significant difference was found in serum GDNF levels in COVID-19 patients and CG. Conclusion: There was no difference in serum NGF and serum GDNF levels in COVID-19 patients compared to the healthy control group.
Keywords:
Nerve growth factor – COVID-19 – coronavirus – glial-derived neurotrophic factor
Introduction
COVID-19, severe acute respiratory syndrome (SARS) or coronavirus 2 (SARS-CoV-2), continues to spread rapidly, affecting the world since December 2019. Although it was known that lungs and heart were among the organs affected by the virus at the beginning, studies have shown the destructive effect of the virus in many systems and organs, including the central (CNS) and peripheral nervous system (PNS) [1].
There are many unclarified questions regarding the neurological symptoms of COVID-19. Headaches, ataxia, impaired consciousness, dizziness, encephalitis, encephalopathy, acute cerebrovascular disease, and CNS diseases such as seizures are among the symptoms. PNS symptoms include loss of taste and smell, visual disturbances and neuromuscular disorders [2]. SARS-CoV-2 was detected in studies performed with genomic sequencing with samples taken from the cerebrospinal fluid (CSF) of COVID-19 patients who developed encephalitis, and it has been shown that the virus can invade the CNS [3]. It is thought that the virus, which is known to invade cells through the angiotensin converting enzyme 2 (ACE2) receptor, which is also expressed by glial cells and neurons, can affect the CNS with the same receptor [1].
Nerve growth factor (NGF) is the first discovered member of the neurotrophin (NT) family. In addition, other NTs consist of brain-derived neurotrophic factor (BDNF), NT-3, and NT-4/5 [4]. NGF is a homodimeric polypeptide and consists of 121 amino acid residues. It is known that NGF has a very important role in processes such as tissue proliferation and differentiation, neuronal angiogenesis/apoptosis and brain development [5]. Another neurotrophic factor identified following NGF is BDNF, which was isolated from the pig brain. It is released from glial cells and glutamatergic neurons in the cortex, hippocampus, hypothalamus and cerebellum, where it is found in high concentrations [6]. Many studies report that BDNF levels are low in diseases associated with loss of cognitive functions, in anxiety, depression, Alzheimer’s disease and schizophrenia etc. [7–11]. In addition to this, the role of glial-derived neurotrophic factor (GDNF) in maintaining the vitality of both dopaminergic neurons and noradrenergic neurons has been emphasized in the recent studies [12]. Monocytes, macrophages, mast cells, T- and B-cells express NGF during the inflammatory response [13]. Neurological symptoms such as disturbance of consciousness, dizziness, headache, etc., which are symptoms of COVID-19, are thought to be related to the production of NFs [14].
Although it is known that the symptoms seen in COVID-19 cases affect the respiratory tract, it has been revealed that the virus affects other systems, especially the nervous system, and sometimes only the nervous system. In our study, we investigated how the COVID-19 disease influenced GDNF and NGF serum levels.
Materials and methods
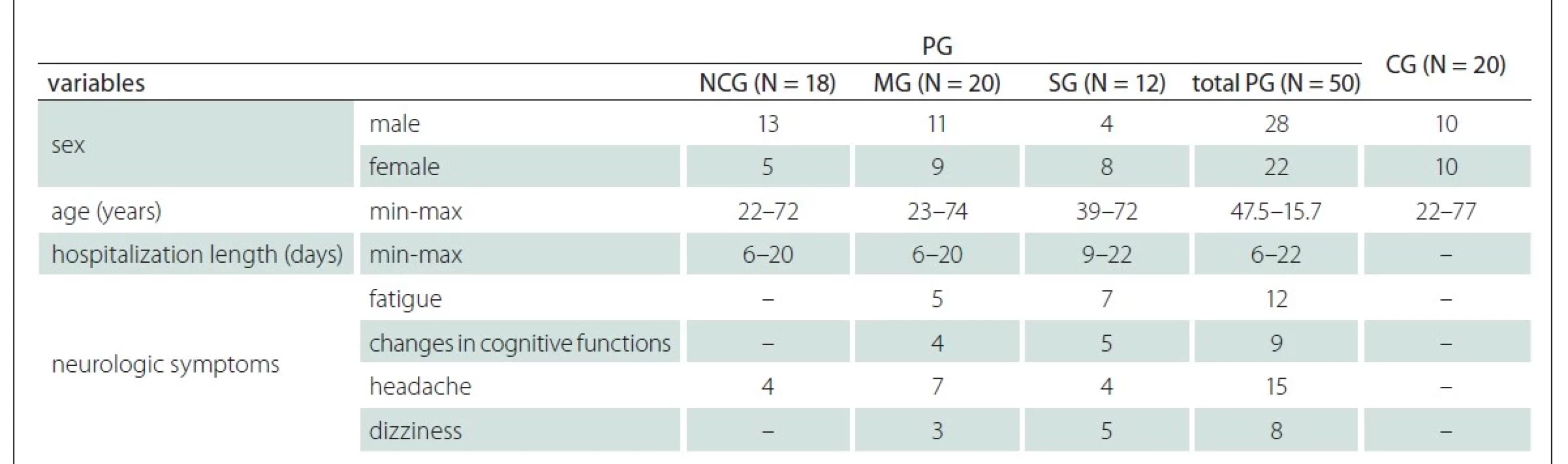
Participants from a previous study formed the sample group for our present investigation [15]. Overall 50 patients (patient group; PG) with a positive reverse transcription polymerase chain reaction (RT-PCR) test for COVID-19 and 20 healthy volunteers (healthy control group; CG) who had no complaints and were negative for COVID-19 RT-PCR were included. All COVID-19 cases were evaluated according to national guidelines. COVID-19 patients were classified according to the criteria given in Tab. 1. On the first day of hospitalization, blood was collected from all patients in the moderately severe group (MG) and severe group (SG). In order to assess symptoms of stress-induced fatigue disorder, participants were interviewed and asked to fill out a self-reporting symptom questionnaire as well as the Karolinska Exhaustion Disorder Scale. The symptom questionnaire includes patients demographic characteristics, length of hospital stay and perceived severity of illness. Patients also reported neurological symptoms such as fatigue, “brain fog” and changes in cognition (i.e., memory or concentration), headache, dizziness.
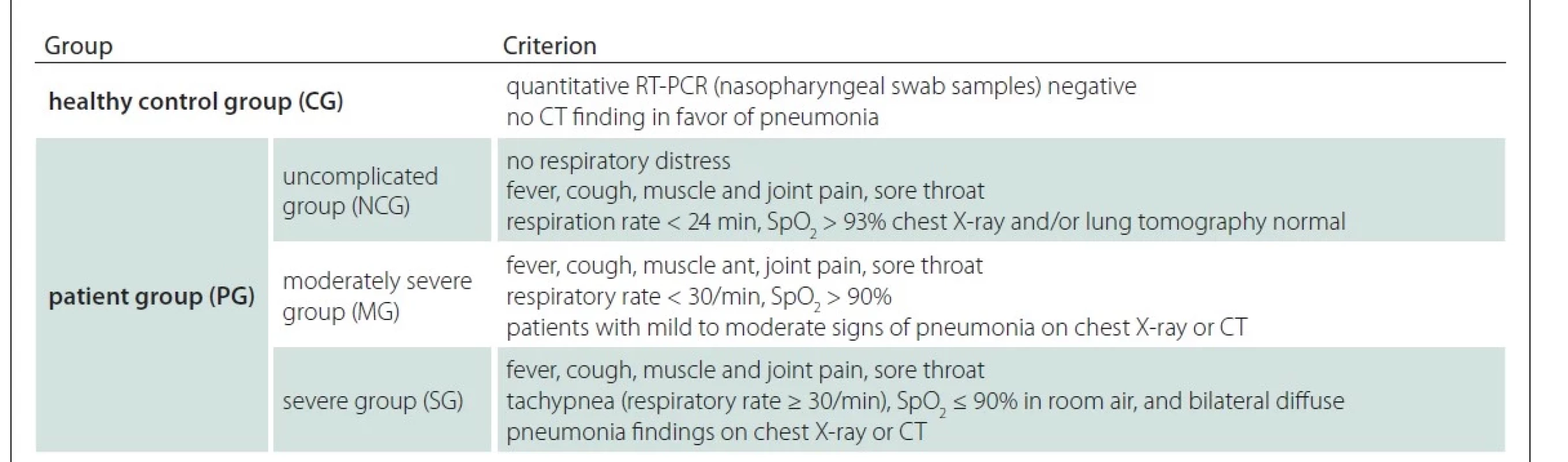
Experimental procedure
Blood samples taken from the patients who applied to the Atatürk University Research Hospital Infectious Diseases Department were sent to the hospital laboratory for routine analysis and also centrifuged and their serum was separated, and all serum samples were stored at –80 °C until the study day.
Serum NGF and GDNF analysis
Serum NGF concentrations were analyzed with the human-specific NGF/NGF-b ELISA kit PicoKine (cat. no. EK0469; Boster Biological Technology, Pleasanton, CA, USA). Serum GDNF concentrations were analyzed with the human-specific GDNF ELISA kit PicoKine (cat. no. EK0362; Boster Biological Technology). All spectrophotometric measurements were done using a microplate reader spectrophotometer BioTek (XS-Wave Power, Winooski, VT, USA). Neutrophils (NE), lymphocytes (LY), and C-reactive protein (CRP) values were evaluated using a clinical chemistry analyzer.
Statistical analysis
The SPSS 21.0 (IBM SPSS Statistics for Windows, Version 20.0. [IBM Corp., Armonk, NY, USA] released 2012) package program was used for statistical analysis. The data were presented as mean, standard deviation, median (minimum-maximum), and number. A Kolmogorov-Smirnov test was used to determine significance and homogeneity of the data. A Student‘s T-test was used in paired group comparisons and an ANOVA test was used in triple group comparisons for normally distributed data. A Pearson correlation test was used for correlation evaluation. The level of statistical significance for all data was P < 0.05.
Results
Demographic characteristics of the PG and CG are shown in Tab. 2. There was no statistical difference between the mean age of the PG and the CG (P = 0.864). 45.7% of participants were females, while 54.3% were males. According to the survey results, 12 out of 50 COVID-19 patients had fatigue, 9 had changes in cognitive functions, 15 had headache and 8 had dizziness (Tab. 2).
There was no statistical difference in serum GDNF and NGF concentrations between the PG and the CG (P = 0.891 and P = 0.86, respectively) (Fig. 1 and 2). Serum NGF levels in the MG subgroup were statistically significantly higher than in the NCG and SG subgroups (P = 0.042). The boxblot graphs of the GDNF and NGF levels in the particular COVID-19 subgroups are shown in Fig. 3 and 4.
CG – healthy control group; GDNF – glial-derived neurotrophic factor;
PG – COVID 19 patient group
Obr. 1. Krabicový graf hladin sérového GDNF u pacientů
s COVID-19 a kontrolní skupiny.
CG – zdravá kontrolní skupina; GDNF – gliální neurotrofní faktor;
PG – pacienti s onemocněním COVID-19

CG – healthy control group; NGF – nerve growth factor;
PG – COVID 19 patient group
Obr. 2. Krabicový graf hladin sérového NGF u pacientů
s COVID-19 a kontrolní skupiny.
CG – zdravá kontrolní skupina; NGF – nervový růstový faktor;
PG – pacienti s onemocněním COVID-19

CG – healthy control group; GDNF – glial-derived neurotrophic factor; MG – moderately severe
group; NCG – uncomplicated group; SG – severe group
Obr. 3. Krabicový graf hladin sérového GDNF u pacientů v podskupinách COVID-19
a kontrolní skupině.
CG – zdravá kontrolní skupina; GDNF – gliální neurotrofní faktor; MG – středně těžká skupina;
NCG – nekomplikovaná skupina; SG – těžká skupina

CG – healthy control group; MG – moderately severe group; NCG – uncomplicated group;
NGF – nerve growth factor; SG – severe group
Obr. 4. Krabicový graf hladin sérového NGF u pacientů v podskupinách COVID-19 a kontrolní
skupině.
CG – zdravá kontrolní skupina; MG – středně těžká skupina; NCG – nekomplikovaná skupina;
NGF – nervový růstový faktor; SG – těžká skupina

No difference was observed between the COVID-19 subgroups and the CG in the NE count. LY count was found to be significantly lower in the PG than in the CG (P = 0.049 and P = 0.001, respectively). In our study, it was observed that as the clinical picture worsened in the COVID-19 subgroups, the CRP level increased and this increase reached a significant difference (P = 0.001). Results of biochemical measurements in all groups are shown in Tab. 3.
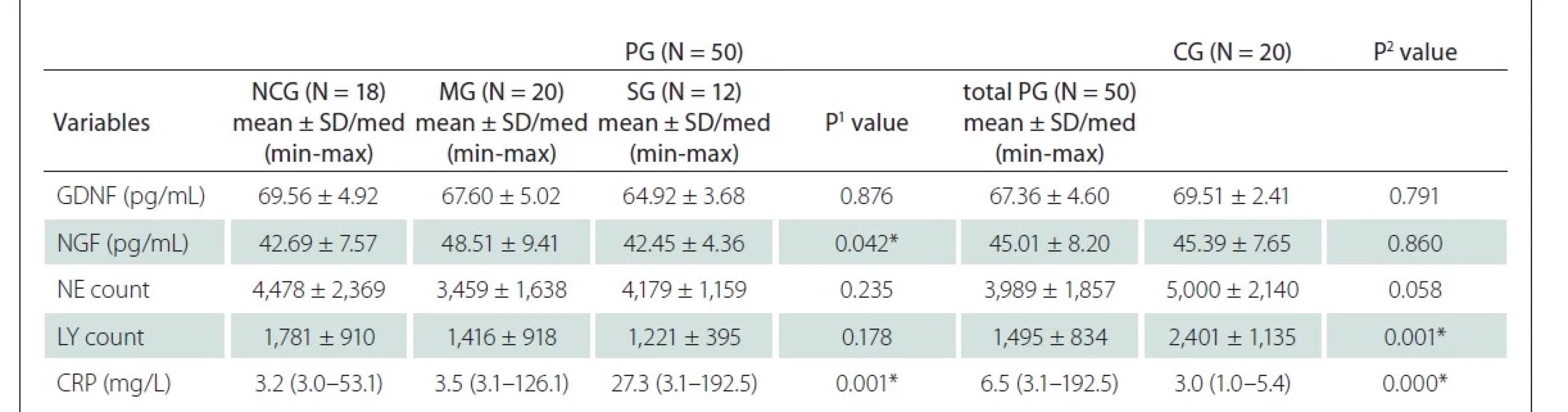
CG – healthy control group; CRP – C-reactive protein; GDNF – glial cell line-derived neurotrophic factor; LY – lymphocyte; MG – moderately
severe group; N – number; NCG – uncomplicated group; NE – neutrophil; NGF – nerve growth factor; PG – COVID 19 patient group; SG – severe
group
Discussion
In the reported cases of COVID-19, it has been reported that the virus affects not only the respiratory tract, but also other organ systems, especially the vascular systems, and most importantly, the nervous system. The symptoms in these cases are quite different from person to person. While the disease does not cause any symptoms in some cases, in some of them, it can be very severe. It is known that GDNF has a neuroprotective effect on dopaminergic and serotonergic neuron survival, preventing oxidative damage and playing a role in neuroinflammation [16]).
In our study, COVID-19 patients were divided into subgroups according to the severity of the disease, and serum GDNF and NGF levels were evaluated in all subgroups. In our study, we found no statistically significant difference in serum GDNF and NGF levels between the PG and the CG. However, serum NGF levels in the MG subgroup were statistically significantly higher than in the NCG and SG subgroups. In addition, among the routine biochemistry tests, the number of LY was significantly lower in the PG.
We found no study in the literature reporting the relationship of neurotrophic factors, such as survival, regeneration and maintenance of specific neuronal populations in the adult brain in COVID-19 patients. However, the relationship between COVID-19 and neurological complications such as smell disorders, encephalopathy, viral meningo- encephalitis and Guillain-Barré syndrome has still not been clarified.
Several studies were reviewed that reported COVID-19 damage to the CNS. In the case study performed by Moriguchi et al, a patient with viral pneumonia developed meningitis with a new onset of epileptic seizures. Brain imaging of the patient revealed right mesial temporal lobe hyperintensity, paranasal sinusitis and hippocampal atrophy. While SARS CoV-2 RNA could not be detected in the nasopharyngeal swab test of the patient, CSF SARS CoV-2 RNA PCR test was positive [17]. This study is very important in terms of showing that COVID-19 cannot be excluded even if the nasopharyngeal swab results are negative in patients with viral pneumonia who develop CNS infection. In another case reported by Gutiérrez-Ortiz C et al., Miller Fisher syndrome was diagnosed in a patient with areflexia of deep tendon reflexes in the upper and lower extremities. In addition, the patient had oculomotor nerve lesion, ophthalmoparesis, and broad-based ataxic gait. In this patient, the nasopharyngeal SARS-CoV-2 virus test was positive. Result of the nasopharyngeal SARS-CoV-2 virus examination was positive in this patients [18]. In meta-analysis, Román et al reported a wide range of neurological diseases in COVID-19 patients, e. g., encephalopathy, meningitis, seizures, Guillain-Barré syndrome, and polyneuropathy [19]. Due to the neuroinvasive nature of COVID-19, it is not yet known how it affects brain functions and what the consequencies of COVID-19 invasion to the CNS might be.
Azouley et al [20] reported that BDNF levels were found to be low in COVID-19 patients, but decreased further with the increasing severity of the disease. Again, in this study, while a negative correlation was observed between BDNF and ferritin levels, the authors found an increase in serum BDNF levels after recovery. Based on previous reports, they concluded that a cytokine storm may have suppressed BDNF.
Our study is the first to evaluate serum NGF and GDNF levels in COVID-19 patients, and we found no statistically significant difference in these levels between COVID-19 patients and healthy controls.
Ethical Approval
This study was approved by the Atatürk University Faculty of Medicine Clinical Research Ethics Committee. (Meeting Number: 05, Decision No: 41, 26. 6. 2021). Informed consent was obtained from all individuals included in this study.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Ozge Nur Türkeri
Department of Pharmacy Services,
Çanakkale Health Services
Vocational School, Çanakkale
Onsekiz Mart University,
Çanakkale, Turkey
e-mail: ozgenur.turkeri@comu.edu.tr
Accepted for review: 22. 6. 2022
Accepted for print: 1. 12. 2022
Zdroje
1. Baig AM, Khaleeq A, Ali U et al. Evidence of the COVID--19 virus targeting the CNS. tissue distribution, host-virus ınteraction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020; 11 (7): 995–998. doi: 10.1021/acschemneuro.0c00122.
2. Mao L, Jin H, Wang M et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA 2020; 77 (6): 683–690. doi: 10.1001/jamaneurol.2020.1127.
3. Lofy KH, Wiesman J, Bruce H et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382 (10): 929–936. doi: 10.1056/NEJMoa 2001191.
4. Friess H, Zhu ZW, Dimola FF et al. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg 1999; 230 (5): 615–624. doi: 10.1097/00000658-199911000-00002.
5. Barichello T, Lemos J, Generoso JS et al. Evaluation of the brain-derived neurotrophic factor, nerve growth factor and memory in adult rats survivors of the neonatal meningitis by Streptococcus agalactiae. Brain Res Bull 2013; 92: 56–59. doi: 10.1016/j.brainresbull.2012.05. 014.
6. Di Carlo P, Punzi G, Ursini G. Brain-derived neurotrophic factor and schizophrenia. Psychiatr Genet 2019; 29 (5): 200. doi: 10.1097/YPG.0000000000000237.
7. Zhang F, Zhu ZQ, Liu DX et al. Emulsified isoflurane anesthesia decreases brain-derived neurotrophic factor expression and induces cognitive dysfunction in adult rats. Exp Ther Med 2014; 8 (2): 471–477. doi: 10.3892/etm.2014.1769.
8. Szuhany KL, Otto MW. Assessing BDNF as a mediator of the effects of exercise on depression. J Psychiatr Res 2020; 123: 114–118. doi: 10.1016/j.jpsychires.2020.02.003.
9. Maass A, Düzel S, Brigadski T et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in order adults. Neuroimage 2016; 131: 142–154. doi: 10.1016/j.neuroimage.2015.10.084.
10. Erickson KI, Voss MW, Prakash RS et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 2011; 108 (7): 3017–3022. doi: 10.1073/pnas.1015950108.
11. Penadés R, López-Vílchez I, Catalán R et al. BDNF as a marker of response to cognitive remediation in patients with schizophrenia: a randomized and controlled trial. Schizophr Res 2018; 197: 458–464. doi: 10.1016/j.schres.2017.12.002.
12. Silva PGC, Domingues DD, Carvalho LA et al. Neurotrophic factors in Parkinson‘s disease are regulated by exercise: evidence-based practice. J Neurol Sci 2016; 363: 5–15. doi: 10.1016/j.jns.2016.02.017.
13. Torcia M, Bracci-Laudiero L, Lucibello M et al. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell 1996; 85 (3): 345–356. doi: 10.1016/s0092-8674 (00) 81113-7.
14. Mathieu VD, Hines DJ, Hines RM et al. Influence of previous COVID-19 and mastitis ınfections on the secretion of brain-derived neurotrophic factor and nerve growth factor in human milk. Int J Mol Sci 2021; 22 (8): 3846. doi: 10.3390/ijms22083846.
15. Özgeriş FB, Koçak ÖF, Kurt N et al. High serum progranulin levels in COVID-19 patients: a pilot study. Biochemistry (Mosc) 2022; 87 (3): 207–214. doi: 10.1134/S000 6297922030026.
16. Henderson CE, Phillips HS, Pollock RA et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science 1994; 266 (5187): 1062–1064. doi: 10.1126/science.7973664.
17. Moriguchi T, Harii N, Goto J et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 2020; 94: 55–58. doi: 10.1016/j.ijid.2020.03.062.
18. Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S et al. Miller-Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020; 95 (5): e601–e605. doi: 10.1212/WNL.0000000000009619.
19. Román GC, Spencer PS, Reis J et al. WFN Environmental Neurology Specialty Group. The neurology of COVID--19 revisited: a proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J Neurol Sci 2020; 414: 116884. doi: 10.1016/j.jns.2020.116884.
20. Azoulay D, Shehadeh M, Chepa S et al. Recovery from SARS-CoV-2 infection is associated with serum BDNF restoration. J Infect 2020; 81 (3): e79–e81. doi: 10.1016/j.jinf.2020.06.038.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2023 Číslo 2
Nejčtenější v tomto čísle
- Current and future therapeutic options for the treatment of the generalized form of myasthenia gravis
- Problematika posuzování invalidity po prodělané cévní mozkové příhodě
- Cenobamát v léčbě farmakorezistentní fokální epilepsie
- Standardizované a pokročilé techniky MR v diagnostice dětských nádorů mozku
