The effects of tacrolimus on cognitive functions in a rat model of cerebral vasospasm
Účinky takrolimu na kognitivní funkce v modelu cerebrálního vazospazmu u potkanů
Cíle: Cílem této studie je prokázat účinnost takrolimu (TAC), látky s imunosupresivními účinky díky inhibici kalcineurinu, v léčbě cerebrálních vazospazmů indukovaných subarachnoidální hemoragií (SAH) na modelu potkana. Materiály a metody: Do studie bylo po získání souhlasu etické komise zahrnuto 18 potkanů (samců) Sprague-Dawley. Potkani mohli v Morrisově vodním bludišti plavat ve čtyřech směrech, dokud nebylo dosaženo plató křivek kognitivních funkcí (4 dny). Po nácviku plaveb byly u potkanů v celkové anestezii fixovány všechny čtyři končetiny a hlava byla držena ve flexi pod úhlem 20° v pronační poloze. SAH byla indukována podáním 0,4 cm3/kg autologní arteriální krve do cisterna magna. Takrolimus (0,5 mg/kg, 2 dávky) byl podán intraperitoneálně pro léčbu indukovaného vazospazmu a byla hodnocena jeho účinnost. Závěr: Při porovnávání časů plaveb za 3 dny a ve všech směrech v kontrolní skupině a skupinách SAH a SAH-TAC bylo pozorováno snížení středních časů plaveb ve skupině, které byl po SAH podán takrolimus. V rámci analýzy rozptylu pomocí Kruskalova-Wallisova testu však nebyly v distribuci středních časů plaveb za tři dny pozorovány žádné signifikantní rozdíly mezi kontrolou a skupinami SAH a SAH-TAC na 95% intervalu spolehlivosti (p = 0,366).
Klíčová slova:
takrolimus – vazospazmus – kognitivní funkce
Authors:
F. Sahinturk; E. Sonmez; N. Altinors
Authors place of work:
Department of Neurosurgery, Baskent University School of Medicine, Ankara, Turkey
Published in the journal:
Cesk Slov Neurol N 2021; 84/117(2): 195-198
Category:
Původní práce
doi:
https://doi.org/10.48095/cccsnn2021195
Summary
Aim: The present study aims to demonstrate the efficacy of tacrolimus (TAC), an agent showing immunosuppressive effects through calcineurin inhibition, in the treatment of subarachnoid haemorrhage (SAH)-induced cerebral vasospasm in rat models. Material and Methods: The study, after gaining the approval of the ethics committee, included 18 male Sprague-Dawley rats which were allowed to swim in four directions within a Morris water maze until the cognitive function curves reached a plateau (4 days). After the retention swimming, four extremities were found while the rats were held in a prone position with the head at 20 degrees flexion under general anaesthesia. SAH was induced through the administration of 0.4 cc/ kg of autologous arterial blood into the cisterna magna. Tacrolimus (0.5 mg/ kg, 2 doses) was administered intraperitoneally for the treatment of the induced vasospasm, and its efficacy was investigated. Conclusion: When the three-day all-direction swimming times of the control, of SAH and SAH-TAC groups were compared, a decrease was noted in the mean times of swimming within the group administered with tacrolimus after SAH, although a Kruskal-Wallis variance analysis did not indicate any significant difference in the distribution of the mean three-day swimming times between control, SAH and SAH-TAC groups, at a 95% confidence interval (P = 0.366).
Keywords:
tacrolimus – vasospasm – cognitive function
Introduction
Subarachnoid haemorrhage (SAH) is the fourth most common cerebrovascular disease after atherothrombosis, embolism and primary intracerebral haemorrhage. Trauma is the most frequently seen cause of SAH, while spontaneous SAH is most commonly caused by the rupture of cerebral aneurysms, which account for 75–80% of all cases [1,2]. Incidences of aneurysmal SAH vary between 7 and 25 per 100,000. Saccular aneurysms are the most commonly seen form of aneurysms, which develop as a result of multifactorial mechanisms.
One of the most significant complications related to SAH is cerebral vasospasm, which is one of the major causes of morbidity and mortality after SAH. The blood collected in the subarachnoid space after aneurysm rupture induces different effects at different time intervals. The release of such side-products as haemoglobin, bilirubin, bilirubin oxidation products, methaemoglobin, free radicals and thromboxane A2 after the haemolysis of the blood in the subarachnoid space leads to the development of a sustained multicomplex response in the organism [3]. Affecting several processes such as oxidation, lipid peroxidation, inflammation, proliferation, and enzyme inhibition and activation, this response creates a cerebral vasospasm.
While the mechanisms through which tacrolimus shows immunosuppressive activity are yet to be fully understood, they are believed to be involved in calcineurin inhibition, the activation of antigen-specific T-cells and the inhibition of release of inflammatory cytokines, mainly interleukin-2 (IL-2), but also others such as IL-4 and IL-5. The calcineurin enzyme is involved in the break of the phosphate groups from target proteins and calcium activation, and plays an essential role in transcriptional activation of cytokines, while also being a good target for immunosuppression. Tacrolimus passes through the cell membrane and binds to the tacrolimus-binding protein, which is a cytosolic protein that is abundant in T lymphocytes. The complex formed by tacrolimus and its binding protein (tacrolimus-binding protein) inhibits calcineurin at sites distant from the phosphate-active sites and physically prevents the interaction of the calcineurin-transcription factor and blocks dephosphorylation. As a result, the synthesis and release of IL-2 and other cytokines are inhibited [4].
The present study aims to demonstrate the efficacy of tacrolimus, an agent showing immunosuppressive effects through calcineurin inhibition, in the treatment of SAH--induced cerebral vasospasm in rat models.
Material and Methods
According to the statistical analysis performed before the study, the sample size that will provide 80% test power at 95% confidence level, with an effect width corresponding to n2 = 0.4 for one-way analysis of variance, which will be used for the difference control between independent groups in terms of numerical variables, is minimal. It was calculated as 18 rats (at least 6 rats in each group).
After the ethics committee’s approval was obtained, a total of 18 male Sprague-Dawley rats weighing approximately 400 g were sourced for the study. Before being allocated to the different study groups, all rats were allowed to practice swimming for four days, different directions every day (east, north, south, west), in a Morris water maze (MWM). The time required for the rats to reach the platform in the water maze was determined as 120 s. The minimum time to be spent on the platform was considered to be 10 s. After the practice swimming, the rats were separated into three groups, the control group (C), the SAH--induced group and the group administered with tacrolimus after SAH (SAH-TAC). The rats in the control group did not undergo any intervention and all of the rats aside from those in the control group were intraperitoneally (i. p.) administered ketamine hydrochloride 50 mg/ kg and xylazin 8 mg/ kg as anaesthetic agents before the procedures.
Intracardial arterial blood samples were obtained under anaesthesia from the rats in the SAH and SAH-TAC groups. The rats were positioned by fixing all four extremities, while the head was held at a 20-degree flexion in the prone position. After the occipitocervical region was sterilised, the foramen magnum was detected through the skin by palpation. The atlantooccipital membrane was entered with a 30 G needle, and SAH was induced through the administration of 0.4 cc of intracardially obtained autologous arterial blood into the cisterna magna.
Following the induced SAH, the rats in the SAH-TAC group were administered 0.5 mg/ kg tacrolimus through thei.p. route on the day of SAH and on the first day after SAH.
On the third day following the induced SAH, the cognitive functions of the rats were evaluated using the MWM, for which the rats in all groups were allowed to swim every day for 3 consecutive days and in all four directions (north, east, south, west). The rats were left in the water at random points and in random order. The duration of the evaluation for each direction was determined as 120 s, and the time taken to reach the platform was recorded. The minimum time to be spent on the platform was determined as 10 s.
Statistical Analysis
The data were analyzed with a “one way variance analysis”, comparing any differences in the numerical variables between the independent groups when the parametric test assumptions were met, and by a Kruskal-Wallis variance analysis when those assumptions were not met. A Shapiro-Wilk normality test was used to evaluate whether each numeric variable planned to be tested between the groups fit into a normal distribution, and a Levene test was used to test if the group variances were homogeneous. Descriptive statistics were presented as mean ± standard deviation for parametric variables and as median (minimum-maximum) values for the non-parametric variables. A Type I error probability was considered á = 0.05 for all analyses. All statistical analyses were carried out using the SPSS v17.0 (IBM, Chicago, IL, USA – September 2012, licence number: 1093910) program.
Results
The time spent swimming in the MWM in four different directions for three days was recorded for all rats in the control group, the induced SAH group and the SAH-TAC group. The mean daily values were recorded in seconds and the swimming time for each rat was recorded by calculating the average swimming time over three days (Tab. 1).
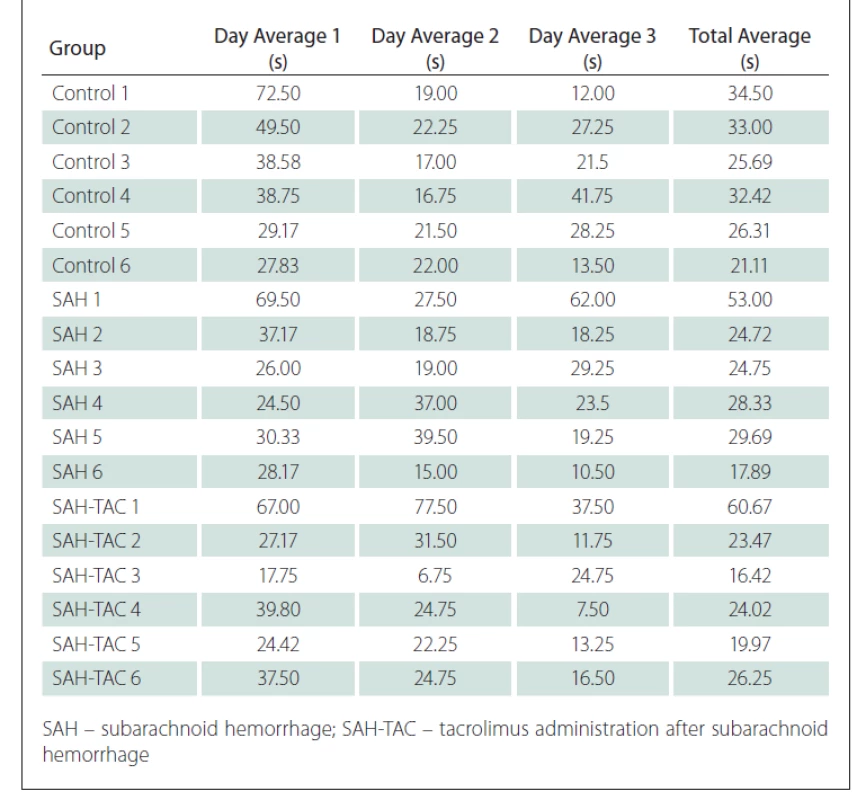
The swimming times decreased every day as the cognitive functions of the rats improved.
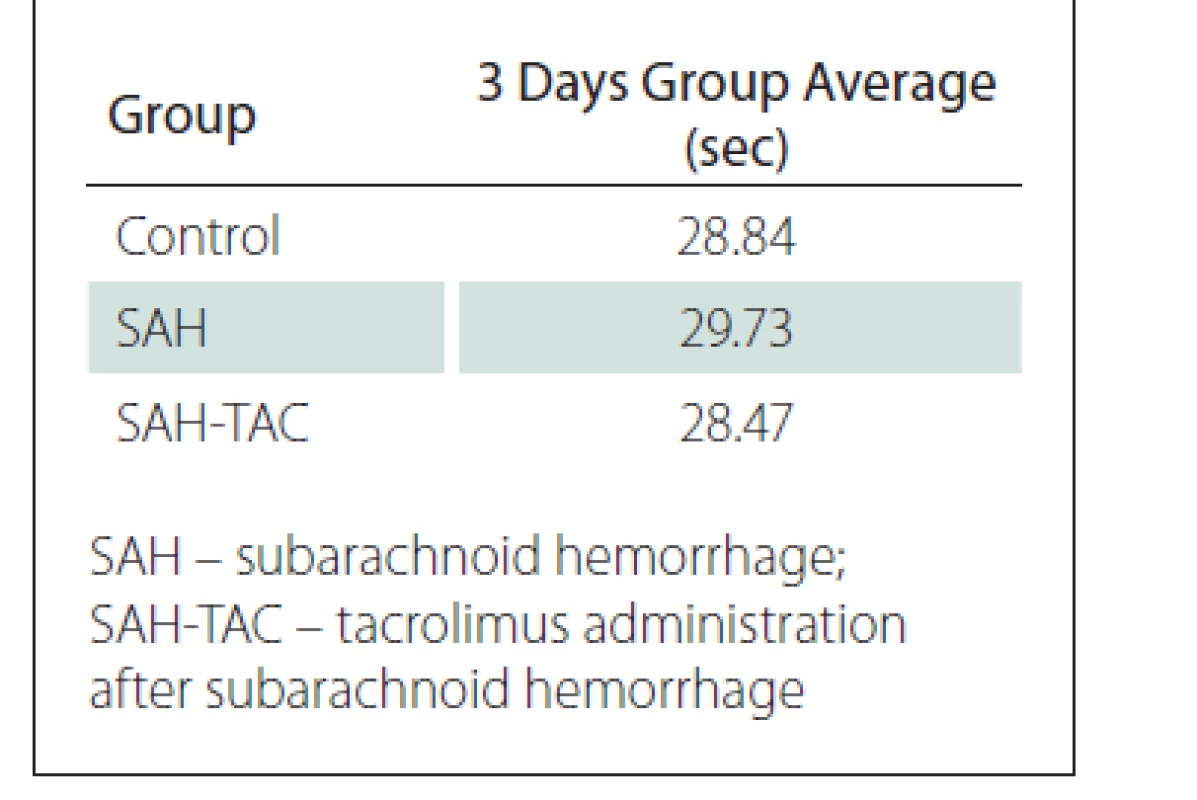
The mean swimming times of the rats in the control, SAH and SAH-TAC groups were calculated based on swimming times of the rats in all four directions for three days (Tab. 2)
At a 95% confidence interval, the Kruskal-Wallis variance analysis did not identify any significant difference between the control, SAH and SAH-TAC groups in terms of the distribution of the mean swimming times recorded on the first day (P = 0.372).
When the mean swimming times recorded in all four directions for three days were compared between the control, SAH and SAH-TAC groups, the mean time was found to be lower in the SAH-TAC group. However, the Kruskal-Wallis variance analysis identified no significant difference within a 95% confidence interval between the control, SAH and SAH-TAC groups in terms of the distribution of the mean three-day swimming times (P = 0.366).
Discussion
The present study demonstrated impairment of cognitive function in the rats due to vasospasm following SAH. Inflammatory mechanisms are activated following the SAH, leading to the development of vasospasm. The presence of oxy-hemolysate in the subarachnoid space activates the inflammatory cascade, and the response to SAH is a complex reaction that results from a breakdown of the products accumulating in the subarachnoid space [3]. Cascade products released during the inflammatory process strongly stimulate vasoconstriction and vascular remodelling [5]. IL-6 plays a key role in the immune response and is responsible for the neurologic presentations that develop during and after vasospasm [5-7]. Huang et al [8], in an experimental study of rats, demonstrated that a cerebral ischaemia following SAH was associated with the severity of the vasospasm and caused diffuse inflammation.
Patients need life-long immunosuppressive therapy following orthotopic liver transplantation (OLT), in which calcineurin inhibitors (tacrolimus or cyclosporine), mycophenolate mofetil and steroids are the most commonly used agents [9].
In the present study, the expected deterioration in cognitive function in rats was noted in the cognitive function curves detected using an MWM water labyrinth in rats in which SAH had been induced. The time taken to locate the platform in the water labyrinth decreased in the group of rats administered with tacrolimus, although no statistically significant difference was found between the particular groups in this regard. This statistical insignificance was considered to be due to the low number of rats in the groups and the fact that an effective vasospasm could not be induced. It can be suggested, however, that tacrolimus, based on its inhibitory effect on cytokine synthesis, suppressed the cytokine response produced following the SAH, thus preventing vasospasm. The decrease in the time taken by the rats who were administered tacrolimus to find the platform in the water labyrinth in this study supports our hypothesis.
Knowledge of the effects of long-term calcineurin inhibitor treatment following OLT on the central nervous system was limited until the 2018 study performed by Pflugrad et al [10] that included 1,045 transplant patients. In the study, both cognitive dysfunction and structural brain changes were reported to be possibly induced in patients undergoing long-term calcineurin inhibitor treatment following the OLT [10].
No dysfunction was found in the cognitive function evaluation performed following the administration of two doses of i.p. tacrolimus (0.5 mg/ kg) in the rats in the SAH-TAC group in the present study, which contradicts the results of the study by Pflugrad et al [10] The difference between the results of these two studies was attributed to the short-term application of tacrolimus treatment and the fact that no long-term effects of the drug were evaluated.
The present study has some limitations. As in previous studies, there are limitations arising from the difficulty of creating effective SAH and vasospasm in rats [11]. The expected statistical results could not be reached due to the difficulties faced in inducing SAH and an efficient vasospasm. Despite carrying out a statistical power analysis prior to the study, more significant results could be obtained by increasing the sample size of the groups. Furthermore, carrying out a histopathological evaluation in addition to the cognitive function evaluation would result in more significant outcomes.
In conclusion, the short-term application of tacrolimus following SAH may protect against cerebral vasospasm and neurologic dysfunction. We suggest similar study with larger sample groups in order to better understand probable beneficial effects of tacrolimus.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008).
The study was approved by the Başkent University Medicine and Health Sciences Research Committee (Project No: DA 18/ 10) and was supported by the Başkent University Research Fund.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products or services used in the study.
Funding
This study has no source of funding.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Fikret Şahintürk, MD
Department of Neurosurgery
Başkent University School of Medicine
A: Fevzi Cakmak Caddesi 10.Sokak No:45
Bahçelievler 06490 Ankara
Turkey
e-mail: fikretsahinturk@gmail.com
Accepted for review: 13. 2. 2020
Accepted for print: 24. 3. 2021
Zdroje
1. Bederson JB, Connolly ES Jr, Batjer HH et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009; 40(3): 994–1025. doi: 10.1161/ STROKEAHA.108.191395.
2. Suarez JI, Zaidat OO, Suri MF et al. Length of stay and mortality in neurocritically ill patients: impact of a specialized neurocritical care team. Crit Care Med 2004; 32(11): 2311–2317. doi: 10.1097/ 01. ccm.0000146132.29042.4c.
3. Dumont AS, Dumont RJ, Chow MM et al. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery 2003; 53(1): 123–133. doi: 10.1227/ 01.neu.0000068863.37133.9e.
4. He XZ, Ma JJ, Wang HQ et al. Brain injury in combination with tacrolimus promotes the regeneration of injured peripheral nerves. Neural Regen Res 2017; 12(6): 987–994. doi: 10.4103/ 1673-5374.208595.
5. Rodriguez-Rodriguez A, Egea-Guerrero JJ, Ruiz de Azua-Lopez Z et al. Biomarkers of vasospasm development and outcome in aneurysmal subarachnoid hemorrhage. J Neurol Sci 2014; 341(1–2): 119–127. doi: 10.1016/ j.jns.2014.04.020.
6. Przybycien-Szymanska MM, Ashley WW Jr. Biomarker discovery in cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 2015; 24(7): 1453–1464. doi: 10.1016/ j.jstrokecerebrovasdis.2015.03.047.
7. Schoch B, Regel JP, Wichert M et al. Analysis of intrathecal interleukin-6 as a potential predictive factor for vasospasm in subarachnoid hemorrhage. Neurosurgery 2007; 60(5): 828–836. doi: 10.1227/ 01.NEU.0000255440. 21495.80.
8. Huang Q, Wang G, Hu YL et al. Study on the expression and mechanism of inflammatory factors in the brain of rats with cerebral vasospasm. Eur Rev Med Pharmacol Sci 2017; 21(12): 2887–2894.
9. Herzer K, Strassburg CP, Braun F et al. Selection and use of immunosuppressive therapies after liver transplantation: current German practice. Clin Transplant 2016; 30(5): 487–501. doi: 10.1111/ ctr.12708.
10. Pflugrad H, Schrader AK, Tryc AB et al. Longterm calcineurin inhibitor therapy and brain function in patients after liver transplantation. Liver Transpl 2018; 24(1): 56–66. doi: 10.1002/ lt.24984.
11. Gules I, Satoh M, Clower BR et al. Comparison of three rat models of cerebral vasospasm. Am J PhysiolHeart Circ Physiol 2002; 283(6): H2551–H2559. doi: 10.1152/ ajpheart.00616.2002.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2021 Číslo 2
Nejčtenější v tomto čísle
- Morton’s neuralgia, metatarsalgia
- Moyamoya disease
- Correct and incorrect naming of pictures for the more demanding written Picture Naming and Immediate Recall test (door PICNIR)
- Gut microbiota and autism spectrum disorders