Prognostic role of neutrophil-lymphocyte ratio in pediatric cystic adamantinomatous craniopharyngioma
Prognostická úloha poměru neutrofilů k lymfocytům u dětského typu cystického adamantinomatózního kraniofaringeomu
Cíl: Bylo prokázáno, že poměr neutrofilů k lymfocytům (NLR) v periferní krvi, což je hematologický indikátor systémového zánětu, je prognostickým faktorem u různých typů nádorového onemocnění. Počet studií, které hodnotí NLR u pacientů s kraniofaryngeomem (CP) je omezený a ani vztah mezi velikostí cystické komponenty a NLR u dětských pacientů s CP nebyl v dostupné literatuře dosud zkoumán. Cílem této studie bylo prozkoumat vztah mezi NLR a velikostí cystické komponenty u dětských pacientů s CP.
Metody: Retrospektivně byly zrevidovány zdravotní záznamy dětských pacientů, kteří byli operováni pro selární/supraselární cystu a jejichž výsledky patologického vyšetření v období leden 2008 až prosinec 2019 prokázaly adamantinomatózní CP. Byla změřena velikost cystické komponenty na předoperační MR a byl stanoven NLR v periferní krvi odebrané pred operací.
Výsledky: Do studie bylo zařazeno 19 pacientů (10 dívek) ve věku 2–17 let (průměr 9 let). Minimální a maximální rozměry cystické komponenty na MR byly 1,12 cm3 a 26,32 cm3. Byla zaznamenána kladná korelace mezi NLR a velikostí cystické komponenty (r = 0,992; p < 0,05), předoperačním výskytem diabetes insipidus (r = 0,624), předoperačním výskytem hydrocefalu (r = 0,825) a subtotální resekcí (r = 0,634). Záporná korelace byla zaznamenána mezi NLR a hrubou totální resekcí (r = −0,634) a skóre dotazníku před operací přední spodiny lebeční (r = –0,729).
Závěr: Velikost cystické komponenty u dětských pacientů s CP je v úzkém vztahu s NLR. Preoperační hodnoty NLR odráží nejen informaci o lokálním zánětu v místě CP, ale poskytuje také představu o velikosti cystické komponenty, závažnosti symptomů a prognóze. Při NLR ≥ 2 je prognóza pravděpodobně nepříznivá.
Klíčová slova:
poměr neutrofilů k lymfocytům – kraniofaryngeom – dětský – velikost cystické komponenty
Authors:
E. Bilgin 1; Y. Gezercan 1; A. İ. Okten 1; Z. Boğa 1; O. Dilek 2
Authors‘ workplace:
Department of Neurosurgery, Adana City Training and Research Hospital, Adana, Turkey
1; Department of Radiology, Adana City Training and Research Hospital, Adana, Turkey
2
Published in:
Cesk Slov Neurol N 2021; 84/117(1): 53-58
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn202153
Overview
Aim: Neutrophil-lymphocyte ratio (NLR) in peripheral blood, a hematological indicator of systemic inflammation, has been shown as a prognocytic factor in various types of cancer. There is a limited number of studies evaluating the NLR in patients with craniopharyngioma (CP), and also the relationship between cystic component size and NLR in pediatric patients with CPs has not been investigated in the current literature before. This study aimed to investigate the relationship between NLR and cystic component size in pediatric patients with CP.
Methods: The medical records of pediatric patients who underwent surgery for a sellar/suprasellar cyst and whose pathology results indicated adamantinomatous CP between January 2008 and December 2019 were retrospectively reviewed. The cystic component size in the preoperative MRI with NLR in the preoperative peripheral blood count were measured.
Results: 19 patients (10 females) – age range 2–17 years (mean 9 years) – were recruited. The minimum and maximum cystic component dimensions in MRI were 1.12 cm3 and 26.32 cm3, respectively. A positive correlation was found between NLR and cystic component size (r = 0.992; P < 0.05), preoperative diabetes insipidus (r = 0.624), preoperative hydrocephalus (r = 0.825), and subtotal resection (r = 0.634). A negative correlation was found between NLR and gross total resection (r = −0.634) and preoperative anterior skull base surgery questionnaire scores (r = −0.729).
Conclusion: Cystic component size and NLR are closely related in pediatric patients with CP. Preoperative NLR can not only reflect local inflammatory information on CP but also provide guidance in cystic component size, severity of symptoms, and prognosis. NLR ≥ 2 may have poor prognosis.
Keywords:
neutrophil-lymphocyte ratio – craniopharyngioma – pediatric – cystic component size
Introduction
Cancer-related inflammation causes disease progression by increasing angiogenesis and proliferation of malignant cells [1]. The neutrophil-lymphocyte ratio (NLR) is a hematological indicator of systemic inflammation and was indicated as a risk factor for worse prognosis in some malignancies [2–4].
Craniopharyngioma (CP), which is histologically benign, originates from the Rather sac and craniopharyngeal canal residue. CP is located in the sellar and/or parasellar region [5]. CP, which constitutes 2–5% of all intracranial tumors, also constitutes 6–13% of all pediatric brain tumors [6]. According to the World Health Organization, CP is pathologically classified as benign grade I neoplasm. Pathologically, there are two types of CP: adamantinomatous and squamous papillary.
Although it is a benign tumor, it has a high affinity for paracellar vital structures due to its aggressive behavior and attitude. Due to this feature, regrowth or recurrence can occur postoperatively [7–10]. Patients may have symptoms such as hypothalamic-pituitary dysfunction or neurological and visual impairments [11]. There are no specific and sensitive serum markers for CPs [12].
Studies showed that inflammation was closely related to CP. The inflammatory response is evaluated based on changes in white blood cells that can be measured using standard tests and changes in neutrophil and lymphocyte levels. Moreover, the NLR has an important role in differential diagnosis of patients with tumors [13–16]. It is an inexpensive and easy method to evaluate NLR inflammation from complete blood cell count [1].
Increased NLR before treatment is a poor prognostic factor in many malignancies such as breast, lung, gastrointestinal, gynecological, and metastatic cancers and glioblastoma multiforme [17–20]. Additionally, increased NLR was proved to be related to poor outcome in non-neoplastic diseases, such as stroke [21]. Although prognostic factors such as age, gender, tumor size, tumor location, and treatment are considered to have a possible relationship with recurrence and quality of life (QOL) in patients with CP, the prognosis of CP is still difficult to predict. Histological findings of CP have common features of degenerative changes and inflammation. As a result of inflammation, the tumor increasingly adheres to the neighboring brain and leads to infiltration, resulting in gross total resection (GTR) of the tumor [20].
This study aimed to investigate the relationship between NLR in pediatric patients with CP and cystic component size. Moreover, it also aimed to investigate the relationship between increased NLR and increased cyst component size and amount of tumor resection and finally effect on prognosis postoperatively.
Materials and methods
Patients and healthy controls
The medical records of pediatric patients who underwent surgery for sellar suprasellar mass between January 2008 and December 2019 and whose pathology results indicated adamantinomatous CP were retrospectively analyzed. Patients with other intracranial disease; those who had infection and head trauma in the past 6 months; those with metabolic syndromes and severe impairment of liver and kidney functions; those with blood-related diseases; those with autoimmune diseases, acute infectious conditions and inflammatory disease, medication use related to inflammatory conditions, history of use of steroids, and hypercortisolic state; and those with severe congenital heart disease were excluded from this study. Erythrocyte sedimentation rate and C reactive protein, procalcitonin, and cortisol levels were measured, and patients with abnormal levels were excluded from the study. One male patient was excluded because he did not meet the inclusion criteria, and two male patients were also excluded because their medical records could not be obtained. Finally, 19 patients were included in this study. When selecting the healthy control group, we reviewed the records of age- and gender-matched healthy pediatric individuals (N = 20) who underwent their annual health examination at the hospital.
Visual evaluation
During ophthalmologic evaluation, all patients were examined by performing computerized visual acuity, visual field examination, and fundoscopic eye examination if clinically appropriate before and after surgery.
Neuroradiological evaluation
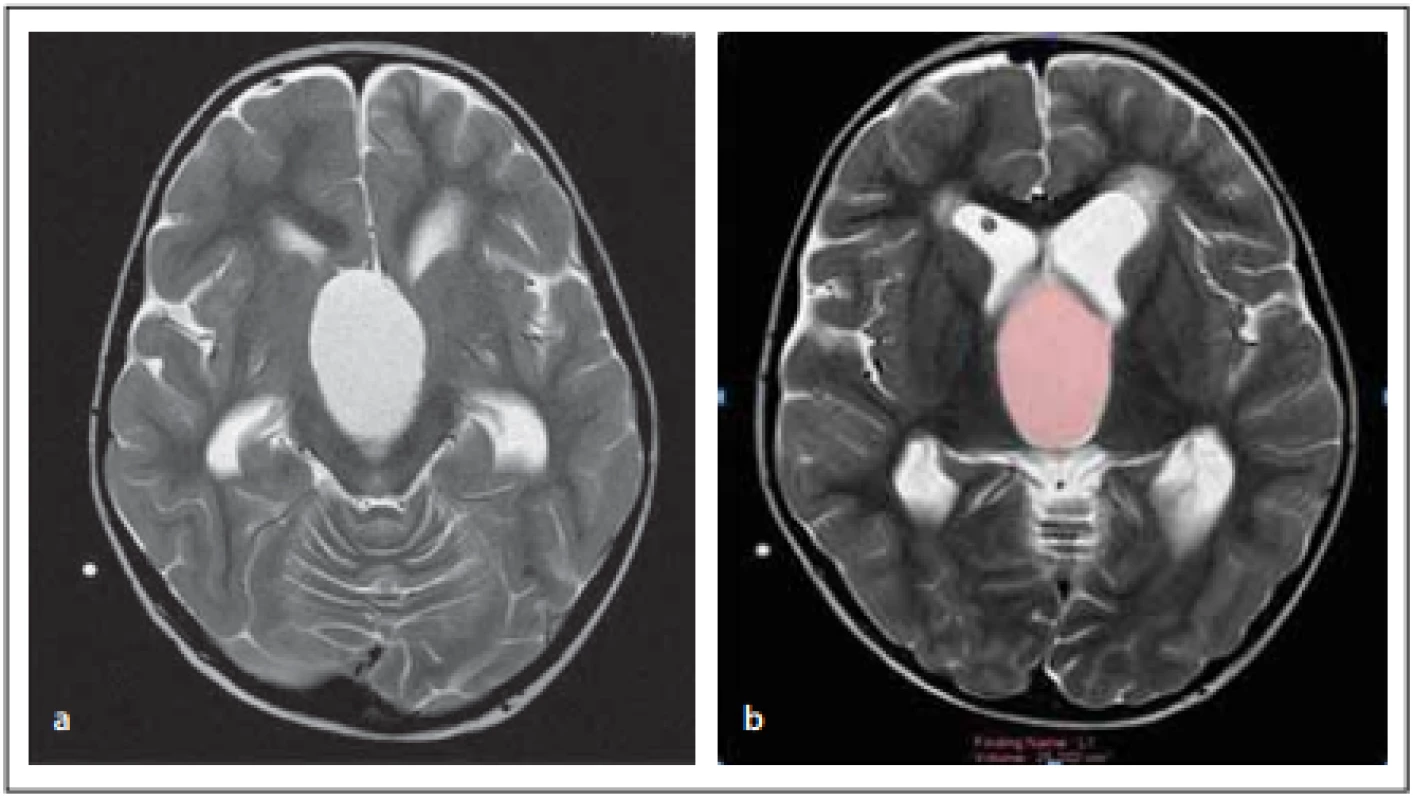
Preoperative brain MRI examinations of patients diagnosed with CP were retrospectively evaluated by experienced radiologists. During the evaluation, the location of lesions was recorded from T2-weighted images. The maximum cystic component sizes of the tumors were measured in three dimensions. The tumor tracking method was used to measure cystic lesions. The evaluations were conducted using the Philips IntelliSpace Workstation System (Amsterdam, Netherlands) (Fig. 1, 2).
Obr. 1. Pacientka (6 let) – (a) cystická komponenta léze na T2 zobrazení v axiální rovině;
(b) měření objemu cystické komponenty léze na T2 zobrazení v axiální rovině.
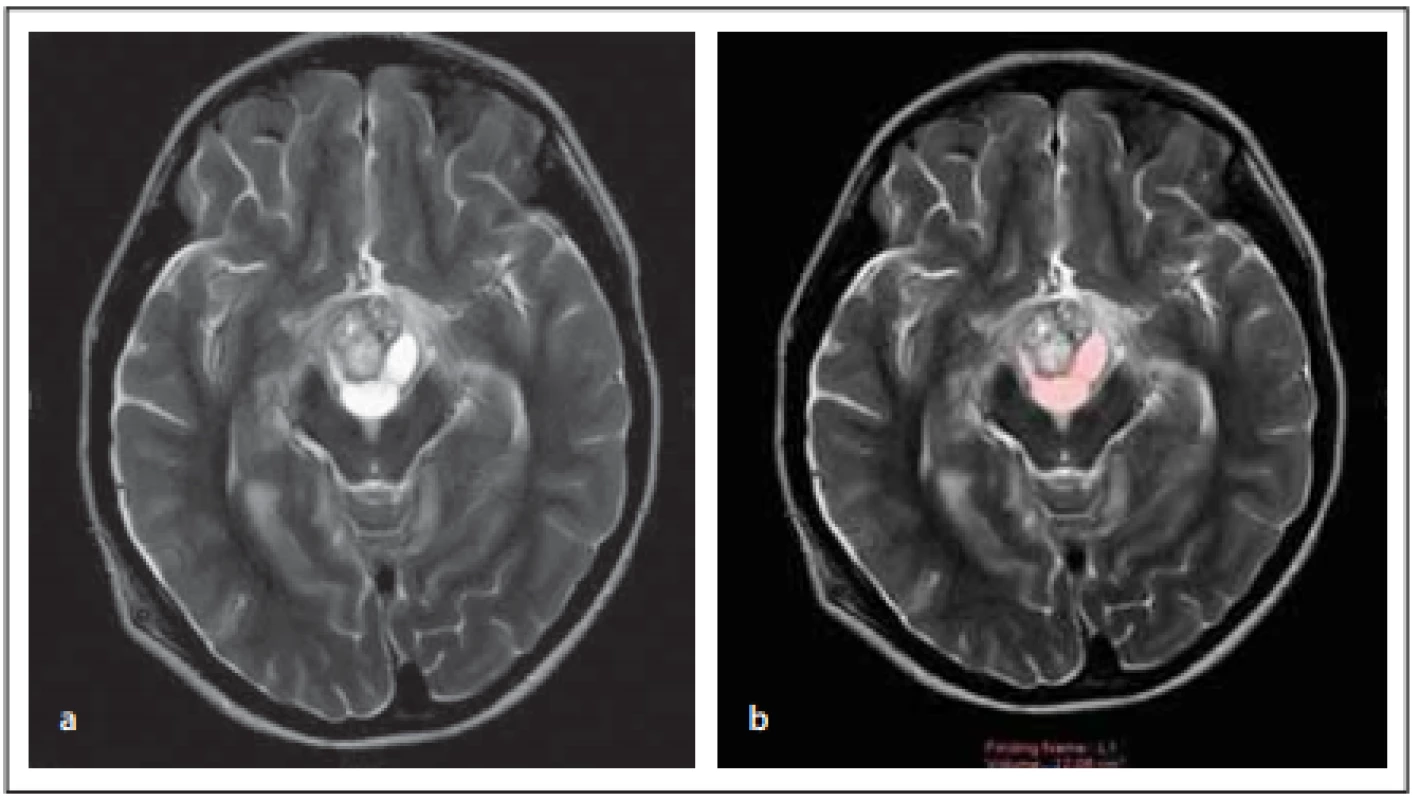
Obr. 2. Pacientka (12 let) – (a) cystická a solidní komponenta léze na T2 zobrazení
v axiální rovině; (b) měření objemu cystické komponenty léze na T2 zobrazení v axiální
rovině.
Surgical techniques
Considering the tumor size and tumor shape, the appropriate approach was selected from the subfrontal and transcortical-transventricular approach as transcranial approaches. While a subfrontal approach was applied only in pediatric patients with CP with suprasellar growth, paraventricular growth, supradiaphragmatic enlargement, or intraventricular growing CP, transcortical-transventricular approach was applied in pediatric patients with CP with other localization and growth patterns.
Data collection
As an anesthetic routine examination 1 week preoperatively, complete blood cell count, hepatic function test, and hormone level test were conducted on blood samples that did not contain any medication and chemotherapy and radiotherapy agent, especially hormone therapy agent such as glucocorticoids. Blood samples were analyzed using a complete blood cell count analyzer (Unicel DxH 800, Coulter Cellular Analysis System [Beckman Coulter, Brea, CA, USA]) within 2 h of blood collection. This study especially focused on blood neutrophil and lymphocyte counts, and the ratio between the two was calculated by comparing the neutrophil count to lymphocyte count (NLR).
Quality of life
For QoL evaluation, The Anterior Skull Base Surgery Questionnaire (ASBS-Q), which included 35 approved questions, was used in patients who underwent anterior skull surgery [22]. Six of these 35 questions aimed to evaluate performance, 7 aimed to evaluate physical function, 7 aimed to evaluate vital signs, 3 aimed to evaluate pain, 5 aimed to evaluate emotions, and finally, 7 aimed to evaluate specific symptoms. The answers provided for each subsection were scored between 1 and 5. The higher the total score is achieved, the better is the QoL of the patients. The score of patients with exitus was 1.
Statistical analysis
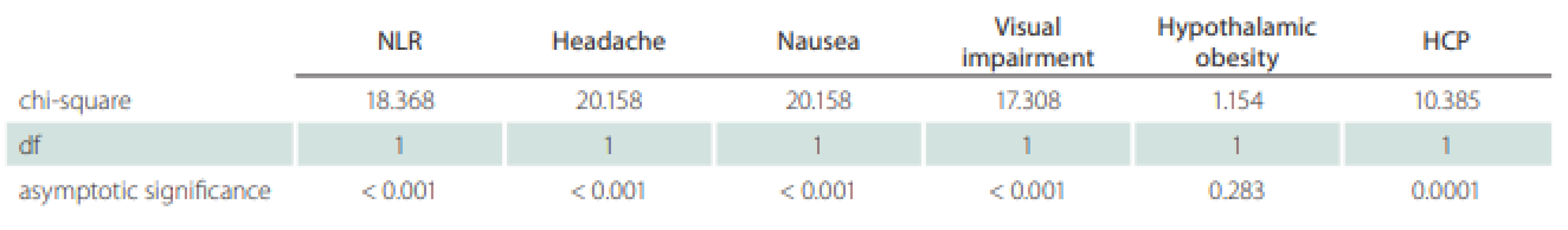
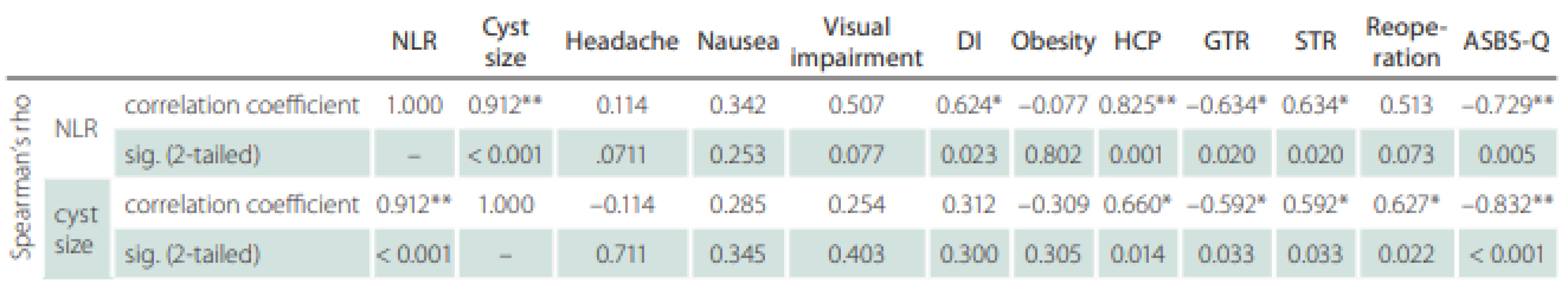
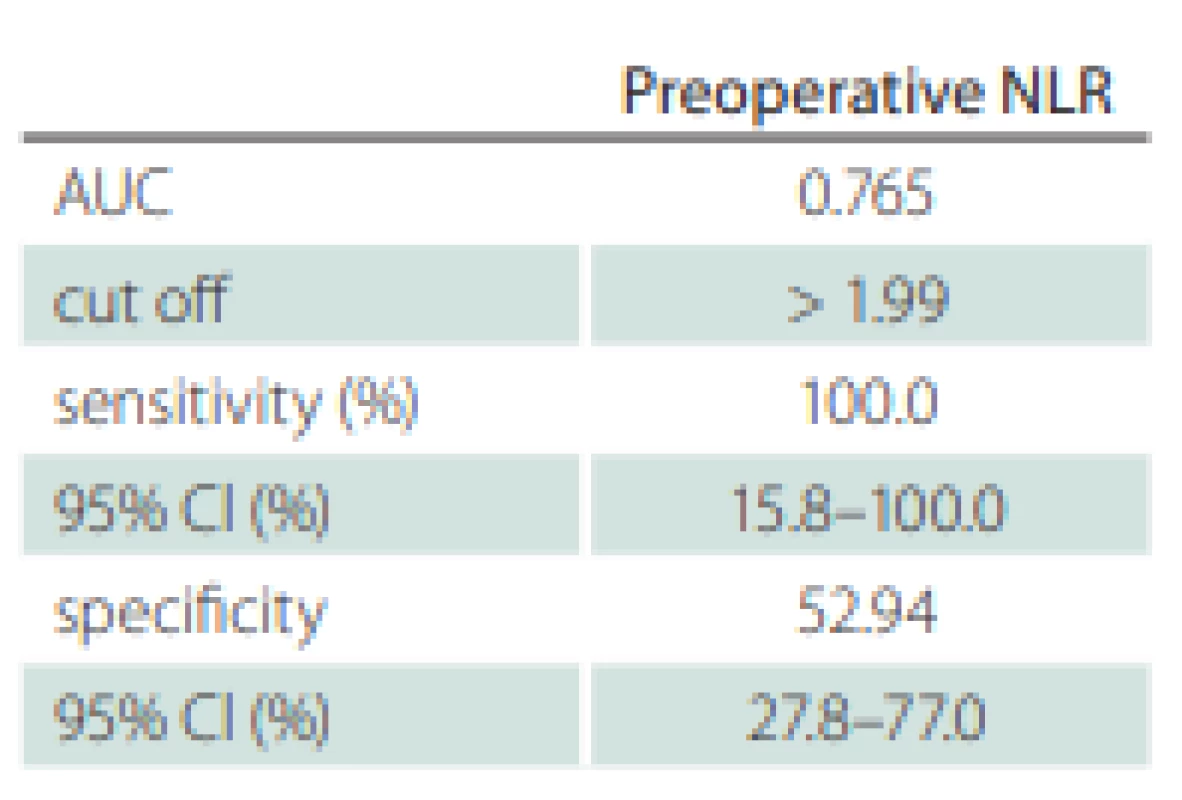
Statistical evaluation of the data was performed using SPSS 23.0 statistical program (SPSS Statistics for Windows, version 23.0. [IBM, Armonk, NY, USA]). The normality of the data was checked using the Kolmogorov-Smirnov test. The degree of statistical significance between datasets (control and patient groups) that showed non-normal distribution was determined using the Kruskal-Wallis test (P < 0.05) (Tab. 1). Correlation levels between different parameters in the same group (patient group) were determined using Spearman’s rho test (P < 0.05) (Tab. 2). Sensitivity and specificity values were calculated based on patients‘ mortality findings and NLRs. Moreover, the area under the receiver operating characteristic curve was analyzed to determine a cutoff value for NLR (Tab. 3).
Results
Patient characteristics
Of 22 patients (12 male and 10 females), one males patient was excluded from the study because he did not meet the inclusion criteria, and two male patients were also excluded because their medical records could not be obtained. The study started with 19 patients (9 male, 10 females; age range 2–17 years; mean age 9 years). The minimum and maximum cystic component dimensions in MRI were 1.12 cm3 and 26.32 cm3 (mean ± standard error, 8.73 ± 2.00), and the minimum and maximum NLRs were 1.02 and 10.55 (mean ± standard error, 3.35 ± 0.63). Ten of 19 (52.63%) patients had elevated NLR (≥ 2) at baseline (Tab. 4).
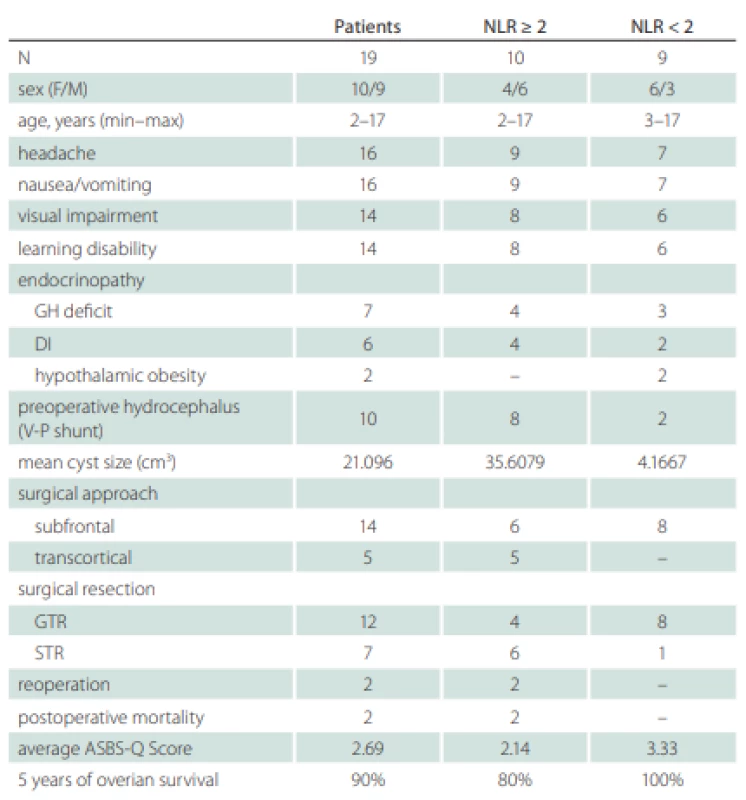
Ten patients underwent ventriculoperitoneal shunt for hydrocephalus (HCP) before tumor surgery. Sixteen patients suffered from headache and nausea, 14 from visual disturbances and learning disability, 7 from growth retardation due to growth hormone deficit, 6 from polyuria and polydipsia due to central diabetes insipidus (DI), and 2 patients from obesity.
When the patient and control groups (N = 20) were compared in terms of preoperative NLRs and severity of symptoms, a significant increase in NLR was noted in pediatric patients with CP compared to the control group. Symptoms of pediatric patients with CP were more severe (P < 0.05).
Postoperative results
Twelve patients underwent GTR, and 7 underwent subtotal resection (STR). Fourteen patients underwent subfrontal approach, and 5 patients underwent transcortical-transventricular approach for mass excision. Due to the recurrence, two patients with transcortical approach underwent the second surgery in 3 and 7 months after the first surgery. Two female patients died due to cardiac problems in the postoperative period. Patients were followed for an average of 90 months (1–120 months). The average ASBS-Q score was 2.69 (Tab. 4).
A positive correlation was found between NLR and cystic component size (r = 0.992; P < 0.05), preoperative DI (r = 0.624), preoperative HCP (r = 0.825), and STR (r = 0.634). A negative correlation was detected between NLR and GTR (r = −0.634) and preoperative ASBS-Q scores (r = −0.729).
A positive correlation was found between cystic component size and preoperative HCP (r = 0.660), STR (r = 0.592), and reoperation (r = 0.627), whereas a negative correlation was found between cystic component size and GTR (r = −0.592) and preoperative ASBS-Q score (r = −0.832).
In pediatric patients with CP, recurrence associated with reoperation correlated with NLR (r = 0.513), and high cystic component size was correlated with reoperation (r = 0.627).
Discussion
Histologically benign CPs are quite difficult intracranial lesions due to their aggressive behavior and attitude [5,7]. They tend to adhere strongly to vital tissues. There is a strong correlation between CP and inflammation, and inflammation has a great influence on the progression and prognosis of CP [23]. In the process of neoplastic development, the result of neutrophil accumulation is associated with inflammation and may be associated with neutrophil tumor reaching high levels in the peripheral blood. Increased inflammation can be the cause for the increased adhesion to the surrounding brain tissue [24].
The cystic part of CP is filled with secreted fluid, cholesterol crystals, and epithelial cells. Despite the presence of benign histological features, the cystic component ratio of CPs is associated with a greater risk of recurrence. For this reason, it is thought to be a proliferative mechanism in its formation and growth [24].
In this study, a positive correlation was found between the inflammatory marker NLR and cystic component size in pediatric patients with CPs, preoperative DI, HCP, and STR. Zhang et al [25] proposed that, when the cutoff NLR was accepted as 4, the risk of mortality increased and the GTR rate was low in patients with NLR ≥ 4, while the long-term results were better in patients with NLR < 4. They reported that NLR was a prognostic factor in patients with CP.
In addition, the cutoff NLR was found as 2, and only 2 patients in the entire patient group died, and these patients were in the group with NLR ≥ 2. Moreover, in patients with NLR ≥ 2, the rate of GTR was lower than that in patients with NLR < 2. This shows that the increasing NLR does not allow GTR of the tumor.
With the increase in inflammation, the GTR decreases and the recurrence rate increases in CPs that gain more adhesive properties to the surrounding brain tissue [26]. Yosef et al [27] reported that recurrence and morbidity were more common in CPs that showed large dimensions. Two patients from the NLR ≥ 2 group in this study were operated for the second time due to the recurrence.
Patients diagnosed with CP are three times more likely to have metabolic syndrome compared to their age-matched controls. Secondary metabolic changes and hypothalamic dysfunction develop due to the surgical procedure and/or tumor [28,29]. Yosef et al [27] reported that only one patient in the small CP group had obesity and stated that this was compatible with the findings of Elliott et al [30]. Obesity was observed in only two patients in our patient group with a small cystic component size and NLR < 2. In the MRI of these obese patients, the solid part of the tumors was larger and suppressed the hypothalamus.
In CPs with large cystic component dimensions, cyst fenestration and excision of the cyst wall may be easier [31]. In this study, although it does not comply with the literature, cyst mouth fixation and cyst excision were more difficult in our patients with NLR ≥ 2 and large cystic component size than in the patient group with NLR < 2 and smaller cystic component size, and GTR could not be performed. This may be caused by the higher adhesion affinity of the cyst to the surrounding brain tissue due to a high NLR.
While the initial symptoms of the increased intracranial pressure (headache) were more frequent in larger CPs (77.7%) compared to smaller CPs (50%), there was no significant difference in preoperative HCP between the two groups [27]. In accordance with the literature, headache was found as an initial symptom in 54.5% of patients with large cystic component size, while HCP was detected more often in patients with larger cystic component size and NLR (85.7%). A positive correlation was found between the cystic component size, NLR, and HCP.
Yaşargil et al [32] proposed that there was a positive correlation between the increasing size of CP and high morbidity or mortality. In a broad comparative study, Elliott et al [30] did not find a significant difference between mortality and small or large CP. Moreover, in this study, preoperative visual impairment was significantly more prominent in the group with larger CP than in the group with smaller CP. While mortality was observed in one patient in the group with NLR ≥ 2 and large cystic component size, there was no mortality in the group with NLR < 2 and small cystic component size. Visual impairment was detected in 85% and 66% of patients with NLR ≥ 2 and NLR < 2, respectively.
Gupta et al reported that the recurrence rate was 58% in CP with tumor size > 4 cm [33]. Another study emphasized that the treatment was not correlated with tumor size in pediatric patients with CPs [34]. Recurrence has not been determined to be correlated with resection yet. In our study, recurrence was detected at 3 and 7 months after STR in 2 patients with NLR ≥ 2 and large cystic component size, and these patients underwent surgery for the second time. Before the second surgery, NLRs of these patients were determined as > 2. The recurrence was correlated with STR, high NLR, and large cystic component size preoperatively.
In our study, the calculated preoperative NLR also significantly differed between pediatric patients with CP and healthy control group. The increase in preoperative NLR may result from an increased neutrophil count or decreased lymphocyte count. This result corresponded to the fact that neutrophil count was elevated in pediatric patients with CP. Unfortunately, the mechanism remaines unknown [35].
The reason for the higher cutoff value in the studies performed by Zhang et al [25] than in our study is that, in this study, a large study group may have been formed by including both adult (papillary) and pediatric (adamantinomatous) CPs. Ming et al [35] formed a large study group by including all CPs (pediatric and adult) and sellar-suprasellar tumors (CPs, renal cell carcinomas, pituitary tumors) in their multicentric study.
Moreover, in these two studies, evaluating both papillary and adamantinomatous CP cases together and not evaluating only pediatric CP alone may be among the reasons for the high NLR.
Preoperative NLR may be associated with better QoL, proper progression-free survival, and even treatment. It is stated that approximately 80% of patients who undergo long-term follow-up after total resection are asymptomatic, and it is emphasized that GTR is associated with better results in the long term [25]. In the QoL evaluation of this study, the ASBS-Q score in the group with NLR ≥ 2 was significantly lower than in the group with NLR < 2. In accordance with the literature, GTR is more commonly applied in the group with high ASBS-Q score.
Because of the single-center and retrospective character of this study, the number of pediatric patients with CP who were included, which is extremely small, and the NLR, which is an indicator of nonspecific inflammation and may produce incorrect positive results, represent its limiting factors.
Conclusion
Inflammation may be closely related to the prognosis of pediatric patients with CP. The preoperative inflammatory marker NLR may be predictive in determining the severity of symptoms, degree of visual impairment and endocrine dysfunction, cystic component size, and amount of surgical resection in pediatric patients with CP as a simple and easily accessible parameter. It should be noted that the symptoms and prognosis will be worse in CPs with NLR ≥ 2 and these patients are a more reasonable group to receive radiation therapy. Improved intracystic therapies based on these results may help inhibit the growth of cyst in pediatric patients with CP. Additional studies are needed to verify our findings.
This study could serve as an initial motivator for large studies to be conducted, along with additional tumor molecular markers or genetic information, as we continue to learn more on pediatric patients with CPs.
Ethical principles
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The authors state that the subjects and their parents provided their written informed consent. The study was approved by the local ethics committee of the Adana City Training and Research Hospital (Turkey) Clinical Researches Ethics Board (October 2020, ref: 68; 1095).
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Emre Bilgin, MD
Department of Neurosurgery
Adana City Training and Research Hospital
Dr. Mithat Özsan Bulvarı Kışla Mah. 4522 Sok. No: 1 Yüreğir
Adana
Turkey
e-mail: dremreblgn@gmail.com
Accepted for review: 12. 11. 2020
Accepted for print: 22. 12. 2020
Sources
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144 (5): 646–674. doi: 10.1016/j.cell.2011.02.013.
2. Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol 2013; 23 (3): 200–207. doi: 10.1016/j.semcancer.2013.02.001.
3. Templeton AJ, McNamara MG, Seruga B et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a system-atic review and meta-analysis. J Natl Cancer Inst 2014; 106 (6): dju 124. doi: 10.1093/jnci/dju124.
4. Guthrie GJ, Charles KA, Roxburgh CS et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013; 88 (1): 218–230. doi: 10.1016/j.critrevonc.2013.03.010.
5. Muller HL. Craniopharyngioma. Handb Clin Neurol 2014; 124: 235–253. doi: 10.1016/B978-0-444-59602-4.00016-2.
6. Mortini P, Losa M, Pozzobon G et al. Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg 2011; 114 (5): 1350–1359. doi: 10.3171/2010.11.JNS10670.
7. Larkin SJ, Ansorge O. Pathology and pathogenesis of craniopharyngiomas. Pituitary 2013; 16 (1): 9–17. doi: 10.1007/s11102-012-0418-4.
8. Martinez-Barbera JP, Buslei R. Adamantinomatous craniopharyngioma: pathology, molecular genetics and mouse models. J Pediatr Endocrinol Metab 2015; 28 (1–2): 7–17. doi: 10.1515/jpem-2014-0442.
9. Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Childs Nerv Syst 2005; 21 (8–9): 622–627. doi: 10.1007/s00381-005-1190-9.
10. Zada G, Lin N, Ojerholm E et al. Craniopharyngioma and other cystic epithelial lesions of the sellar region: a review of clinical, imaging, and histopathological relationships. Neurosurg Focus 2010; 28 (4): E4. doi: 10.3171/2010.2.FOCUS09318.
11. Karavitaki N, Cudlip S, Adams CB et al. Craniopharyngiomas. Endocrine Rev 2006; 27 (4): 371–397. doi: 10.1210/er.2006-0002.
12. Kros JM, Mustafa DM, Dekker LJ et al. Circulating glioma biomarkers. Neuro Oncol 2015; 17 (3): 343–360. doi: 10.1093/neuonc/nou207.
13. Deng Q, He B, Liu X et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med 2015; 13: 66. doi: 10.1186/s12967-015-0409-0.
14. Gasparyan AY, Ayvazyan L, Mikhailidis DP et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011; 17 (1): 47–58. doi: 10.2174/138161211795049804.
15. Gu L, Li H, Chen L et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and metaanalysis. Oncotarget 2016; 7 (22): 31926–31942. doi: 10.18632/oncotarget.7876.
16. Raffetti E, Donato F, Castelnuovo F et al. The prognostic role of systemic inflammatory markers on HIV-infected patients with non-Hodgkin lymphoma, a multicenter cohort study. J Transl Med 2015; 13: 89. doi: 10.1186/s12967-015-0446-8.
17. Han S, Liu Y, Li Q et al. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer 2015; 15: 617. doi: 10.1186/s12885-015-1629-7.
18. Walsh SR, Cook EJ, Goulder F et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005; 91 (3): 181–184. doi: 10.1002/jso.20329.
19. Keizman D, Gottfried M, Ish-Shalom M et al. Pretreatment neutrophilto-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with ketoconazole: association with outcome and predictive nomogram. Oncologist 2012; 17 (12): 1508–1514. doi: 10.1634/theoncologist.2012-0125.
20. Vidal S, Kovacs K, Lloyd RV et al. Angiogenesis in patients with craniopharyngiomas: correlation with treatment and outcome. Cancer 2002; 94 (3): 738–745. doi: 10.1002/cncr.10281.
21. Gokhan S, Ozhasenekler A, Mansur Durgun H et al. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. Eur Rev Med Pharmacol Sci 2013; 17 (5): 653–657.
22. Gil Z, Fliss DM. Quality of life in patients with skull base tumors: current status and future challenges. Skull Base 2010; 20 (1): 11–18. doi: 10.1055/s-0029-1242 979.
23. Chen M, Zhang Z, Yang M et al. Prediction of calcification tendency in pediatric cystic adamantinomatous craniopharyngioma by using inflammatory markers, hormone markers, and radiological appearances. Childs Nerv Syst 2019; 35 (7): 1173–1180. doi: 10.1007/ s00381-019-04178-0.
24. Pettorini BL, Inzitari R, Massimi L et al. The role of inflammation in the genesis of the cystic component of craniopharyngiomas. Childs Nerv Syst 2010; 26 (12): 1779–1784. doi: 10.1007/s00381-010-1245-4.
25. Zhang J, He M, Liuz Z et al. Impact of neutrophil-lymphocyte ratio on long term outcome in patients with craniopharyngioma. Medicine (Baltimore) 2018; 97 (37): e12375. doi: 10.1097/MD.0000000000012375.
26. Kawamata T, Kubo O, Hori T. Histological findings at the boundary of craniopharyngiomas. Brain Tumor Pathol 2005; 22 (2): 75–78. doi: 10.1007/s10014-005-0191-4.
27. Yosef L, Ekkehard KM, Shalom M. Giant craniopharyngiomas in children: short- and long-term implications. Childs Nerv Syst 2016; 32 (1): 79–88. doi: 10.1007/s00381-015-2961-6.
28. Simoneau-Roy J, O’Gorman C, Pencharz P et al. Insulin sensitivity and secretion in children and adolescents with hypothalamic obesity following treatment for craniopharyngioma. Clin Endocrinol 2010; 72 (3): 364–370. doi: 10.1111/j.1365-2265.2009.03639.x.
29. Elowe-Gruau E, Beltrand J, Brauner R et al. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab 2013; 98 (6): 2376–2382. doi: 10.1210/jc.2012-3928.
30. Elliott RE, Wisoff JH. Surgical management of giant pediatric craniopharyngiomas. J Neurosurg Pediatr 2010; 6 (5): 403–416. doi: 10.3171/2010.8.PEDS09385.
31. Zuccaro G. Radical resection of craniopharyngioma. Childs Nerv Syst 2005; 21 (8–9): 679–690. doi: 10.1007/ s00381-005-1201-x.
32. Yasargil MG, Curcic M, Kis M et al. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg 1990; 73 (1): 3–11. doi: 10.3171/jns.1990.73.1.0003.
33. Gupta DK, Ojha BK, Sarkar C et al. Recurrence in craniopharyngiomas: analysis of clinical and histological features. J Clin Neurosci 2006; 13 (4): 438–442. doi: 10.1016/j.jocn.2005.05.013.
34. Kim SK, Wang KC, Shin SH et al. Radical excision of pediatric craniopharyngioma: recurrence pattern and prognostic factors. Childs Nerv Syst 2001; 17 (9): 531–537. doi: 10.1007/s003810100458.
35. Ming C, Shi-hao Z, Min Y et al. The diagnostik value of preoperative inflammatory markers in craniopharyngioma: a multicenter cohort study. J Neurooncol 2018; 138 (1): 113–122. doi: 10.1007/s11060-018-2776-x.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2021 Issue 1
Most read in this issue
- Frontotemporal dementia
- Encephalocele in the Czech Republic – incidence, prenatal diagnostics and international comparison
- COVID-19 and stroke
- Carotid endarterectomy after intravenous thrombolysis and mechanical thrombectomy
