The role of cell adhesion molecules (ICAM-1 and VCAM-1) in acute ischemic stroke
Role adhezních molekul (ICAM-1 a VCAM-1) při akutní ischemické cévní mozkové příhodě
Cíl: Cerebrální ischemie je spojena s infiltrací zánětlivých buněk do ischemické oblasti. Buněčné adhezní molekuly zprostředkovávají shromažďování leukocytů v místě infarktu. Cílem studie bylo prozkoumat spojitost mezi buněčnými adhezními molekulami a akutní ischemickou cévní mozkovou příhodou, neurologickým postižením, cerebrální ischemií a klinickými výsledky u pacientů s cévní mozkovou příhodou. Materiál a metody: Do studie bylo zařazeno 153 pacientů rozdělených do dvou skupin: pacienti s akutní ischemickou cévní mozkovou příhodou a pacienti s rizikovými faktory, kteří cévní mozkovou příhodu neměli. Byly odebrány vzorky krve s cílem analyzovat hladinu intercelulární adhezní molekuly 1 (intercellular adhesion molecule 1; ICAM-1) a vaskulární buněčné adhezní molekuly 1 (vascular cell adhesion molecule 1; VCAM-1) v séru. Ke stanovení neurologické disability u pacientů s cévní mozkovou příhodou byla použita škála National Institutes of Health Stroke Scale. Během prvních 24 hodin po nástupu cévní mozkové příhody byla provedena CT mozku a byly zaznamenány důkazy časných ischemických lézí. Případy cévní mozkové příhody byly rozděleny do podskupin podle klasifikace Trial of Org 10172 in Acute Stroke Treatment (TOAST). Výsledky: U pacientů s cévní mozkovou příhodou byla zaznamenána významně vyšší hladina VCAM-1. Mezi podtypy cévní mozkové příhody a hladinou VCAM-1 nebyla žádná souvislost. Na základě hladiny VCAM-1 jsme byli schopni predikovat stupeň neurologického deficitu s 28,6% přesností. Zjistili jsme významný vztah mezi hladinou VCAM-1 a výskytem ischemických lézí. U pacientů s hladinou VCAM-1 > 740 ng/ml bylo 3,45x vyšší riziko ischemických lézí na CT. U hladiny VCAM-1 nebyla mezi dobou testování séra a klinickým výsledkem prokázána žádná souvislost. Závěr: Naše zjištění svědčí o tom, že v případě akutní cévní mozkové příhody by VCAM-1 mohla sloužit jako biomarker. Hladinu ICAM-1 v séru nelze jako biomarker pro diagnózu a prognózu ischemické cévní mozkové příhody použít.
Klíčová slova:
biomarkery – ischemická cévní mozková příhoda – buněčné adhezní molekuly – VCAM-1 – ICAM-1
Authors:
M. Peycheva 1; T. Deneva 2; Z. Harizanova 3; D. Zlatareva 4,5
Authors place of work:
Department of Neurology, Medical University Plovdiv, Bulgaria
1; Department of Clinical Laboratory, Medical University Plovdiv, Bulgaria
2; Department of Human Anatomy, Histology and Embryology, Medical University Plovdiv, Bulgaria
3; Department of Imaging Diagnostics, Medical University Sofia, Bulgaria
4; Translational Neuroscience Complex, Medical University Plovdiv, Bulgaria
5
Published in the journal:
Cesk Slov Neurol N 2022; 85(2): 168-174
Category:
Původní práce
doi:
https://doi.org/10.48095/cccsnn2022168
Summary
Aim: Cerebral ischemia is associated with the infiltration of inflammatory cells to the ischemic region. Cell adhesion molecules mediate the recruitment of the leukocytes to the infarct zone. The study aimed to explore the associations between cell adhesion molecules and acute ischemic stroke, neurological impairment, cerebral ischemia and clinical outcome of stroke patients. Materials and methods: The study involved 153 patients categorized into two groups: patients with acute ischemic stroke and patients with risk factors who did not have a stroke. Blood samples were collected to analyze the serum level of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). National Institutes of Health Stroke Scale was used to determine the neurological disability of stroke patients. Cerebral CT was performed during the first 24 h after stroke onset and evidences of early ischemic lesions were recorded. The stroke cases were divided into subgroups according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification. Results: A significantly higher level of VCAM-1 was observed in stroke patients. There was no relation between VCAM-1 level and stroke subtypes. Based on VCAM-1 level, we were able to predict the degree of neurological deficit with 28.6% precision. We found a significant relationship between VCAM-1 level and the presence of ischemic lesions. Patients with VCAM-1 levels > 740 ng/mL had a 3.45-fold increased risk of ischemic lesions on CT. VCAM-1 levels showed no association with the time of the serum test and the clinical outcome. Conclusion: Our findings suggest that VCAM-1 might serve as a biomarker for acute ischemic stroke. The serum levels of ICAM-1 cannot be used as a biomarker for the diagnosis and prognosis of ischemic stroke.
Keywords:
ischemic stroke – biomarkers – cell adhesion molecules – VCAM-1 – ICAM-1
Introduction
Cerebral ischemia is associated with the infiltration of inflammatory cells to the ischemic region [1–3]. Cell adhesion molecules (CAMs) are immunoglobulin-like structures which function is to maintain, organize and mediate the migration of leucocytes to the vessel wall, followed by stable adhesion and migration into it [4,5]. Representatives of this group are intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), with a proven role in endothelial dysfunction and atherosclerosis processes [6]. The study aimed to explore the associations between CAMs and acute ischemic stroke, neurological impairment, cerebral ischemia and short-term clinical outcome of stroke patients.
Materials and methods
An observational, cross-sectional study has been conducted for 6 months (from November 2017 through May 2018) and included 153 patients under medical observation at the Department of Neurology, Medical University Plovdiv, Bulgaria. The inclusion criteria were risk factors for ischemic stroke, such as a history of arterial hypertension, carotid atherosclerosis, diabetes mellitus, atrial fibrillation and cardiovascular disease (ischemic heart disease, valvular heart disease, heart failure and others). The exclusion criteria were autoimmune diseases, cancer and inflammatory diseases. The selection was made by means of medical history, physical examination and laboratory investigations. Patients were divided into two main groups: patients with acute ischemic stroke who were not treated with recombinant tissue plasminogen activator (rt-PA) and patients with risk factors but without a history of ischemic stroke. Stroke patients were selected not to be treated with reperfusion therapies, as in the literature there are data that thrombolytic agents could alter the serum concentration of CAMs within the first 24 h [7]. The recruitment of patients from the second group was done at the outpatient clinic and included patients with vascular risk factors. The information about comorbidity in all patients was collected through medical history, physical examination, laboratory investigations, electrocardiogram monitoring and extracranial carotid duplex ultrasound investigation. Venous blood samples (4.5 mmol/ l blood, Monovette [Sarstedt, Nümbrecht, Germany]) from all patients were taken in the morning between 6–8 a. m. as atraumatically as possible. The samples were centrifuged at 2,500 rpm for 10 min and stored at –20 °C until the time of the analysis, but not longer than 2 months, according to the manufacturer’s instructions. The manners of withdrawal, processing and storage of blood samples and the choice of biologic materials were following the requirements and the recommendations given by the manufacturer to compensate for the factors of result variation and for standardization of the preanalytical stage. Serum concentrations of sICAM-1 and sVCAM-1 were determined using ELISA assay (Bender MedSystems, Vienna, Austria) after its local validation. In the literature, there are no standard reference ranges for VCAM-1 and ICAM-1.
In stroke subgroup, the time between the stroke onset and the blood sample taking was recorded. The time of blood collection after the stroke ranged between 6 to 48 h with a median of 24 h. The National Institutes of Health Stroke Scale (NIHSS) was used to determine the neurological disability of stroke patients on hospital admission and at discharge. Short-term clinical outcome (with improvement, without change, with deterioration) was analyzed in stroke patients based on the dynamics of the neurological deficit assessed by NIHSS. Cerebral CT was performed in the same group of patients during the first 24 h after stroke onset and evidences of early ischemic lesions (evidences for hypo-attenuating brain tissue, obscuration of lentiform tissue, dense middle cerebral artery sign, insular ribbon sign, loss of sulcal effacement, loss of gray-white interface, early mass effect) were recorded. The stroke cases were divided into subgroups according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification [8]. In the non-stroke group, the blood tests were done on the day of the other investigations.
Data were analyzed using the statistical software SPSS (IBM, Armonk, NY, USA), Version 25 and Med-Calc Statistical Software (MedCalc, Ostend, Belgium), Version 18.11.3. The continuously measured variables were screened for normality using the Kolmogorov- Smirnov test. The normally distributed ones were described through the mean values and standard deviations (SD), whereas the non-normally distributed ones were presented with the medians and interquartile ranges (IQR). To examine significant differences between the two groups, we performed independent sample t-tests when normality was observed (Kolmogorov- Smirnov p > 0.05) and the Mann Whitney U test when normality was not present (Kolmogorov- Smirnov p < 0.05). Comparisons of more than two groups of normally distributed variables were performed through one-way analysis of variance (ANOVA), and when normality was not observed through the Kruskal-Wallis test. The diagnostic potential of ICAM-1 and VCAM-1 was evaluated through the receiver operating characteristic (ROC) curve analysis. The optimal criterion was used to recode VCAM-1 into positive and negative cases and to calculate the odds ratio (OR) with the incidence of ischemic lesions evident on cerebral CT. For stroke patients, we examined the association between ICAM-1 and VCAM-1 and the neurological deficit measured by NIHSS, the time of CAMs blood testing, and stroke outcome at hospital discharge using a correlation analysis. Pearson correlation analysis was employed when variables were measured continuously and were normally distributed, and Spearman correlation was used in all other cases. Frequency data were presented as numbers and percentages and when they were relevantly examined using the Fisher’s exact test. The results were interpreted as statistically significant if the p values were < 0.05.
Results
Demographic and clinical characteristics of the patients
The study involved 153 patients with a mean age of 65.08 ± 10.65 years, of whom 71 (46.4%) were females (Tab. 1). Seventy-nine patients (51.63%) had an acute ischemic stroke and 74 (48.37%) did not have an ischemic stroke. The mean age, sex distribution, presence of carotid atherosclerosis, arterial hypertension, and diabetes did not differ significantly between stroke and non-stroke patients. A significantly higher percentage of the patients with ischemic stroke had atrial fibrillation and cardiovascular diseases. VCAM-1 levels were significantly higher in stroke patients, whereas ICAM-1 levels did not differ significantly between the two groups.
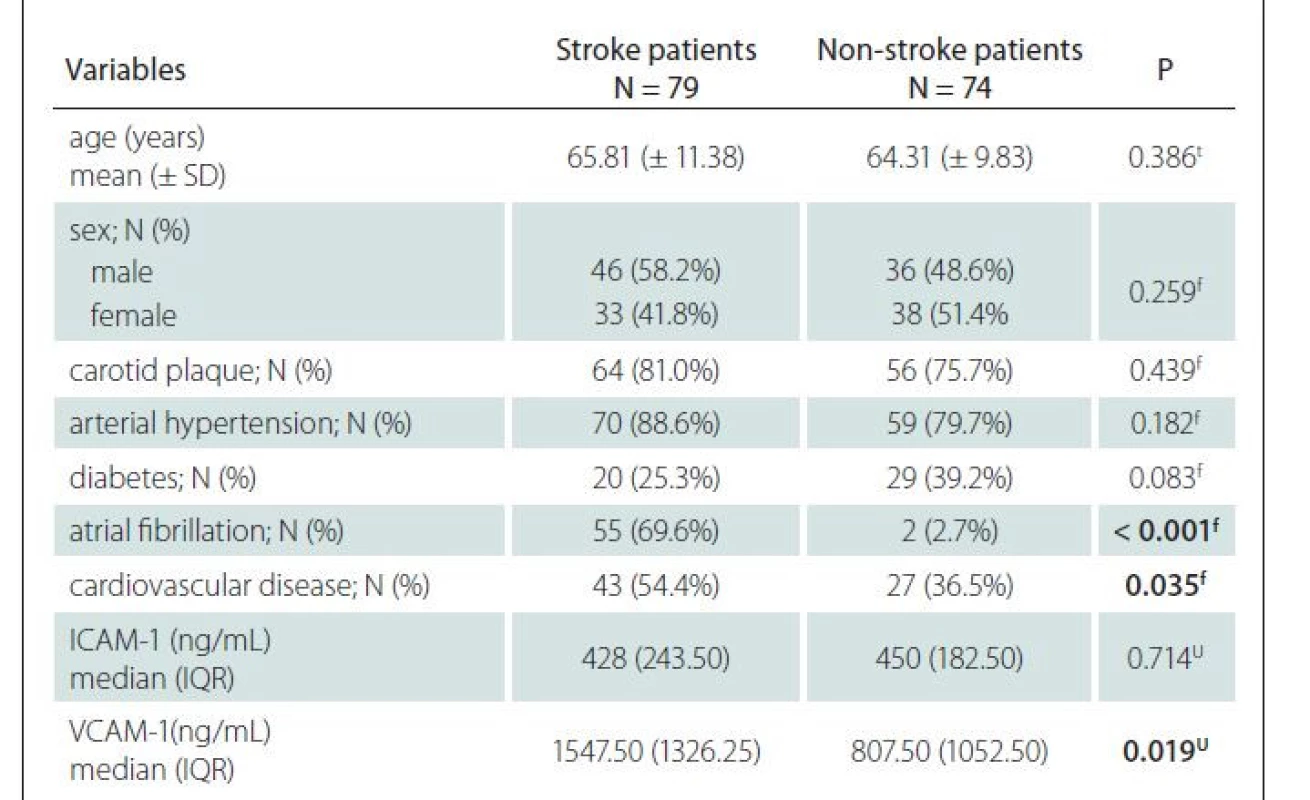
ICAM-1 – intercellular adhesion molecule 1; IQR – interquartile range; N – number; SD – standard
deviation; VCAM-1 – vascular cell adhesion molecule 1
Analysis of ICAM-1 and VCAM-1 levels in particular subtypes of ischemic stroke
To investigate variations in ICAM-1 levels depending on the subtype of stroke according to the TOAST classification, we performed a comparative analysis using the Kruskal- Wallis test. The results showed no statistically significant association between the level of ICAM-1 and the subtype of stroke (P = 0.801) (Fig. 1).
VCAM-1 – vascular cell adhesion molecule 1
Obr. 1. Hodnoty mediánů hladin proteinu VCAM-1.
VCAM-1 – vaskulární buněčná adhezní molekula 1 (vascular cell adhesion molecule 1)

The median levels of VCAM-1 showed a wider variation across ischemic stroke subtypes (Fig. 2) with the highest value (1,970 ng/ mL) observed in atherosclerotic stroke and the lowest one in stroke from another cause (850 ng/ mL); however, the statistical analysis showed no significant difference (P = 0.276). We consider this is due to the small sample size of the stroke-subtype subgroups and suggest that increasing the sample size will increase the likelihood of finding a significant association between stroke-subtype and VCAM-1 levels.
ICAM-1 – intercellular adhesion molecule 1
Obr. 2. Hodnoty medianů hladin proteinu ICAM-1.
ICAM-1 – intercelulární adhezní molekula 1 (intercellular adhesion molecule 1)

Analysis of the relationship between ICAM-1/ VCAM-1 levels and early ischemic lesions on cerebral CT scan
We investigated the diagnostic potential of ICAM-1 and VCAM-1 levels as indicators of ischemic lesions using the receiver operating characteristic (ROC) curve analysis. The results (Fig. 3) showed a lack of prognostic ability ofICAM-1 (AUC = 0.546; P = 0.517) and a weak but significant relationship between VCAM-1 values and the presence of early ischemic lesions on cerebral CT scan taken within the first 24 h from the stroke onset (AUC = 0.637; 95% CI: 0.501–0.772; P = 0.019). The associated criterion value distinguishing patients with ischemic lesions from patients without ischemic lesions was VCAM-1 level > 740 ng/ mL (95% CI: 670– 1,140 ng/ mL) with sensitivity of 78% and specificity of 58%.
AUC – area under curve; ICAM-1 – intercellular adhesion molecule 1; ROC – receiver operating
characteristic; VCAM-1 – vascular cell adhesion molecule 1
Obr. 3. Křivky ROC pro diagnostický potenciál VCAM-1 a ICAM-1 jako indikátorů ischemických
lézí.
AUC – plocha pod křivkou; ICAM-1 – intercelulární adhezní molekula 1 (intercellular adhesion
molecule 1); ROC – receiver operating characteristic; VCAM-1 – vaskulární buněčná adhezní
molekula 1 (vascular cell adhesion molecule 1)

Based on the criterion value (VCAM-1 level > 740 ng/ mL), we categorized patients into two groups, positive (VCAM-1 level > 740 ng/ mL) and negative (VCAM-1 level ≤ 740 ng/ mL), and calculated the odds ratio (OR) of CT ischemic lesions with VCAM-1 seropositivity. The results showed a 3.45-times higher risk of ischemic lesions in patients with VCAM-1 values > 740 ng/ mL (OR = 3.45; 95% CI: 1.5–7.92; P = 0.003).
Analysis of the relationship between ICAM-1 and VCAM-1 levels, neurological deficit, time of the blood test, and clinical outcome at hospital discharge in patients with ischemic stroke
Evaluation of the relationship between ICAM-1 and VCAM-1 levels with neurological deficit (NIHSS), time of the blood test sampling (hours after stroke onset) and clinical outcome at hospital discharge in patients with ischemic stroke (improvement, without change, deterioration) was carried out using a correlation analysis (Tab. 2). The results showed no significant correlations between the levels of ICAM-1 and the three observed variables. Regarding VCAM-1, we found a significant positive linear relation with neurological deficit (NIHSS), while for the other two variables, no significant relationship was found.
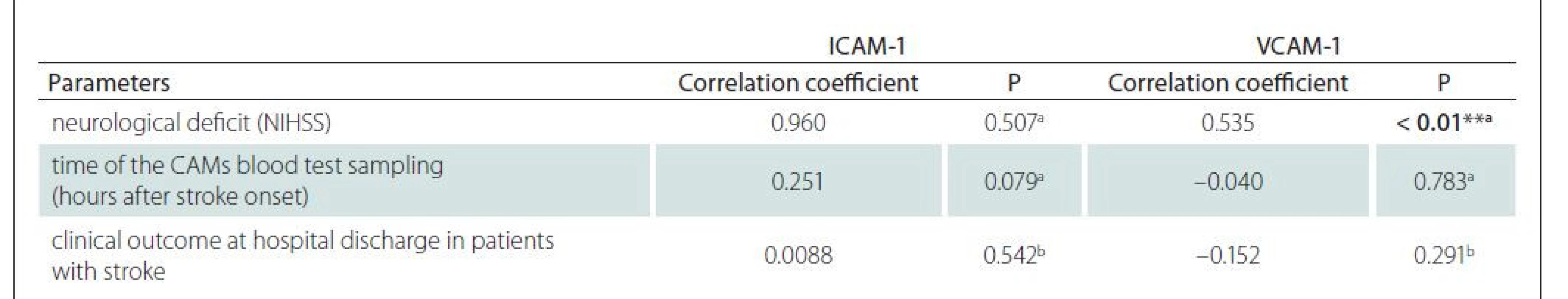
Figure 4 illustrates the positive linear relationship between VCAM-1 levels and neurological deficit. Higher VCAM-1 values were associated with higher NIHSS values and vice versa, with R-square = 28.6% and adjusted coefficient of determination R-square (adj.) = 27.1%.
NIHSS – National Institutes of Health Stroke Scale; VCAM-1 – vascular cell adhesion molecule 1
Obr. 4. Významný lineární vztah mezi hladinou VCAM-1 a neurologickým deficitem.
NIHSS – National Institutes of Health Stroke Scale; VCAM-1 – vaskulární buněčná adhezní molekula 1 (vascular cell adhesion molecule 1)

Discussion
Inflammation plays an important role in the pathogenesis of ischemic stroke and the recruitment of inflammatory cells appears to exacerbate ischemic brain injury [4]. Cell adhesion molecules are involved in the recruitment of leucocytes into post-ischemic brain tissue, with their adhesion and migration into endothelial cells [4,5,9].
In order to investigate the relationship between CAMs (ICAM-1 and VCAM-1) and ischemic stroke, we analyzed the serum concentrations in two main target groups (patients with acute ischemic stroke and patients with risk factors, but without experienced cerebrovascular accident) and traced their distribution to different etiological stroke subtypes, neurological deficits (NIHSS), time of blood tests sampling and short-term clinical outcome after stroke.
Our study showed no association of ICAM-1 level with ischemic stroke, so it cannot be used as a reliable biomarker for stroke diagnosis and prognosis. No correlations were found between ICAM-1 levels and the etiological subtype of strokes. Regarding neurological deficit, time of blood tests sampling, and short term outcome after stroke, serum ICAM-1 levels did not show a statistically significant relationship. In the context of ischemic stroke, ICAM-1 is one of the most studied CAMs [10]. Studies on the dynamics of serum ICAM-1 levels in the first hours after stroke showed contradictory results. Numerous independent studies found an increase within the first 24 h after the accident [11–13]. Another study comparing stroke patients with healthy controls proved no dependence [14]. The results regarding the relationship between ICAM-1 levels and the outcome of ischemic stroke are also contradictory. Two studies did not show a correlation with ICAM-1 values and stroke severity in the first week, 10 days, and 3 months after the accident [14,15]. According to Sotgiu et al [12], high levels of ICAM-1 at the beginning of the accident were associated with more extensive cerebral infarctions and a poorer 3-month prognosis. Wang et al [16] found that the serum level of sICAM-1 on admission was associated with neurological deterioration in ischemic stroke.
VCAM-1 is the other type of CAM with a proven role in the pathophysiological mechanisms of ischemic stroke [10]. Studies comparing patients with and without stroke found higher serum VCAM-1 values in the stroke group [12,14]. In our study, we also observed significantly higher levels of sVCAM-1 in stroke patients compared to the control group of patients with vascular risk factors, who did not experience cerebrovascular accident. The mean value of sVCAM-1 in patients with ischemic stroke was 1,516.60 ± 947 ng/ mL. Although there are still no generally accepted reference ranges for CAMs, if we use the data obtained by Deneva et al [17] for the values of VCAM-1 (170.42–478.36 ng/ mL) in healthy controls for the Bulgarian population, we see that the levels obtained by us are significantly above the normal range.
Despite the lack of statistical significance, the level of VCAM-1 showed a definite relationship with the subtypes of stroke, with the highest value observed in strokes due to atherosclerosis of the large arteries. This fact seems logical, given the role of CAMs in the processes of endothelial dysfunction and atherogenesis [6,18,19].
Supanc et al [14] studied the role of CAMs in different etiological subtype of stroke. They found increased levels of VCAM-1 in thromboembolic events and a lack of such dependence in lacunar strokes. Serum VCAM-1 values in thromboembolic events ranged from 90–1,810 ng/ mL with a median of 730 ng/ mL. In our study, we observed higher mean VCAM-1 values in strokes due to atherosclerosis 1,953.21 ± 1,372 ng/ mL with a median of 1,907 (715–6,055) ng/ mL. The authors also examined the relationship with stroke severity and clinical outcome after the accident, finding no dependencies.
According to some researchers, CAMs in stroke could also affect the size of cerebral infarction [10,13]. On this basis, various experimental drugs have been developed aimed at blocking the function of CAMs and thus reducing the infarct area, as well as improving the neurological deficit [20–24]. Studies proved that anti-adhesion molecules reduced ischemic damage after transient but not after permanent middle cerebral artery occlusion [4,22,25]. This observation suggests that anti-adhesion interventions may offer more therapeutic benefits in patients receiving rt-PA to achieve reperfusion following ischemic stroke [4,26]. In our study, we investigated the diagnostic potential of ICAM-1 and VCAM-1 levels as indicators of ischemic lesions on cerebral CT within 24 h from stroke onset. The results showed a lack of prognostic ability of ICAM-1 and a weak but significant relationship between VCAM-1 values and the presence of ischemic lesions. In the current set, patients with a VCAM level > 740 ng/ mL showed a 3.45-fold increased risk of ischemic lesions (with a sensitivity of 78% and a specificity of 50%).
Our results proved a linear relationship between VCAM-1 values and the degree of neurological deficits determined by NIHSS in stroke patients. Based on VCAM-1 levels, we can predict the degree of neurological deficits with a predictive accuracy of 28.6% for this set and 27.1% if the prognosis is applied to other set. Recent study proved that increased levels of systemic and intracranial VCAM-1 at the time of mechanical thrombectomy could predict ischemic stroke severity assessed using NIHSS [27]. In our study, the blood samples are taken within the first 48 h and VCAM-1 levels did not show a relationship with the time of serum sampling and with the short-term clinical outcome. In a longitudinal prospective study Bitsch et al [28] found that in patients with stroke, VCAM-1 levels reached pathoa maximum after 5 days (P = 0.02). According to Blum et al [29], measuring cell adhesion molecule levels within the first 4 days may objectively predict the clinical outcome in hospitalized patients with acute ischemic stroke.
Conclusion
We can conclude that VCAM-1 might serve as a reliable marker for acute ischemic stroke, especially concerning ischemic lesions and neurological deficits.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). The study protocol was approved by the Local Ethics Committee (31.03.2016/ protocol No2). All subjects provided written informed consent prior to their participation in the study.
Funding
“Markers of inflammation associated with the nature and instability of plaques in carotid atherosclerosis” funded by the Medical University Plovdiv, Bulgaria (project code 04/ 2015). “Cerebrovascular disease – new methods for diagnosis and prevention” funded by Medical University Plovdiv, Bulgaria (project code 17/2020).
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Accepted for review: 3. 1. 2022
Accepted for print: 24. 3. 2022
Marieta Peycheva
Department of Neurology
Medical University Plovdiv
Peshtersko shosse 66 Bul
Plovdiv 4000
Bulgaria
e-mail: mpeitcheva@yahoo.com
Zdroje
1. Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87(5): 779–789. doi: 10.1189/jlb.1109766.
2. Jin R, Liu L, Zhang S et al. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res 2013; 6(5): 834–851. doi: 10.1007/s12265- 013-9508-6.
3. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17(7): 796–808. doi: 10.1038/nm.2399.
4. Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res 2008; 30(8): 783–793. doi: 10.1179/174313208X341085.
5. Schmidt EP, Kuebler WM, Lee WL et al. Adhesion molecules: master controllers of the circulatory system. Compr Physiol 2016; 6(2): 945-973. doi: 10.1002/cphy. c150020.
6. De Graba T. Expression of inflammatory mediators and adhesion molecules in human atherosclerotic plaque. Neurology 1997; 49 (5 Suppl 4): S15–S19. doi: 10.1212/ wnl.49.5_suppl_4.s15.
7. Kerner T, Ahlers O, Reschreiter H et al. Adhesion molecules in different treatments of acute myocardial infarction. Crit Care 2001; 5(3): 145–150. doi: 10.1186/cc1 014.
8. Adams HP Jr, Bendixen BH, Kappelle LJ et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24(1): 35–41. doi: 10.1161/01.str.24.1.35.
9. Radi ZA, Kehrli ME Jr, Ackermann MR. Cell adhesion molecules, leukocyte trafficking, and strategies to reduce leukocyte infiltration. J Vet Intern Med 2001; 15(6): 516–529. doi: 10.1892/0891-6640(2001)015<0516:camlta >2.3.co;2.
10. Ramiro L, Simats A, García-Berrocoso T et al. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther Adv Neurol Disord 2018; 11: 1756286418789340. doi: 10.1177/1756286418789340.
11. Licata G, Tuttolomondo A, Di Raimondo D et al. Immuno-inflammatory activation in acutecardioembolic strokes in comparison with other subtypes of ischaemic stroke. Thromb Haemost 2009; 101: 929–937.
12. Sotgiu S, Zanda B, Marchetti B et al. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol 2006; 13: 505–513. doi: 10.1111/j.1468- 1331.2006.01280.x.
13. Tuttolomondo A, Di Sciacca R, Di Raimondo D et al. Plasma levels of inflammatory and thrombotic/fibrinolytic markers in acute ischemic strokes: relationship with TOAST subtype, outcome and infarct site. J Neuroimmunol 2009; 215: 84–89. doi: 10.1016/j.jneuroim. 2009.06.019.
14. Supanc V, Biloglav Z, Kes V et al. Role of cell adhesion molecules in acute ischemic stroke. Ann Saudi Med 2011; 31(4): 365–370. doi: 10.4103/0256-4947.83217.
15. Orion D, Schwammenthal Y, Reshef T et al. Interleukin- 6 and soluble intercellular adhesion molecule-1 in acute brain ischaemia. Eur J Neurol 2008; 15(4): 323–328. doi: 10.1111/j.1468-1331.2008.02066.x.
16. Wang JY, Zhou DH, Li J et al. Association of soluble intercellular adhesion molecule 1 with neurological deterioration of ischemic stroke: The Chongqing Stroke Study. Cerebrovasc Dis 2006; 21(1–2): 67–73. doi: 10.1159/000090005.
17. Deneva-Koycheva T, Vladimirova-Kitova L, Angelova E et al. Serum levels of sICAM-1, sVCAM-1, sE-selectin, sP-selection in healthy Bulgarian people. Folia Medica 2011; 53(2): 22–28. doi: 10.2478/v10153-010-00 33-y.
18. Endres M, Laufs U, Merz H et al. Focal expression of intercellular adhesion molecule-1 in the human carotid bifurcation. Stroke 1997; 28(1): 77–82. doi: 10.1161/01. str.28.1.77.
19. Iiyama K, Hajra L, Iiyama M et al. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 1999; 85(2): 199–207. doi: 10.1161/01.res.85.2. 199.
20. Becker K, Kindrick D, Relton J et al. Antibody to the α4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke 2001; 32(1): 206–211. doi: 10.1161/01.str.32.1.206.
21. Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 2001; 57(8): 1428–1434. doi: 10.1212/wnl.57.8.1428.
22. Zhang R, Chopp M, Jiang N et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke 1995; 26(8): 1438–1443. doi: 10.1161/01.str.26.8.1438.
23. Bowes MP, Zivin JA, Rothlein R. Monoclonal antibody to the ICAM-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp Neurol 1993; 119(2): 215–219. doi: 10.1006/exnr.1993. 1023.
24. Chopp M, Li Y, Jiang N et al. Antibodies against adhesion molecules reduce apoptosis after transient middle cerebral artery occlusion in rat brain. J Cereb Blood Flow Metab 1996; 16(4): 578–584. doi: 10.1097/0000 4647-199607000-00007.
25. Prestigiacomo CJ, Kim SC, Connolly ES et al. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke 1999; 30(5): 1110–1117. doi: 10.1161/01.str.30.5.1110.
26. Bowes MP, Rothlein R, Fagan SC et al. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology 1995; 45(4): 815–819. doi: 10.1212/wnl.45.4.815.
27. Maglinger B, Sands M, Frank JA, at al. Intracranial VCAM1 at time of mechanical thrombectomy predicts ischemic stroke severity. J Neuroinflammation 2021; 18(1): 109. doi: 10.1186/s12974-021-021 57-4.
28. Bitsch A, Klene W, Murtada L et al. A longitudinal prospective study of soluble adhesion molecules in acute stroke. Stroke 1998; 29(10): 2129–2135. doi: 10.1161/01. str.29.10.2129.
29. Blum A, Khazim K, Merei M et al. The stroke trial – can we predict clinical outcome of patients with ischemic stroke by measuring soluble cell adhesion molecules (CAM)? Eur Cytokine Netw 2006; 17(4): 295–298.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2022 Číslo 2
Nejčtenější v tomto čísle
- Targeted surgery for obstructive sleep apnea
- Balance disorders in patients with multiple sclerosis and possible rehabilitation therapy – current findings from controlled clinical trials
- Successful nonsurgical management of lumbar radiculopathy associated with disc herniation and instability in low back pain syndrome
- Music therapy in voice and speech disorders in patients with Parkinson‘s disease
