Does leptin have a role in the development of intracranial meningiomas?
Hraje leptin roli v rozvoji intrakraniálních meningeomů?
Úvod: Meningeomy jsou nejčastějšími nezhoubnými nádory nitrolební dutiny a tvoří přibližně 30 % všech nitrolebních nádorů. Velká většina z nich je skutečně nezhoubných, avšak určitá podmnožina těchto nádorů se projevuje vyšším výskytem recidivy a nepříznivou mírou morbidity a mortality. Leptin, produkt obézního (ob) genu, je polypeptid o hmotnosti 16 kDA, který se nachází na lidském chromozomu 7. Hraje zásadní roli při regulaci tělesné hmotnosti řízením příjmu potravy, energetického metabolismu a neuroendokrinní funkce.
Cíl: Hledání možného vztahu mezi leptinem a tvorbou intrakraniálního meningeomu. Byla použita prospektivní kontrolovaná klinická studie.
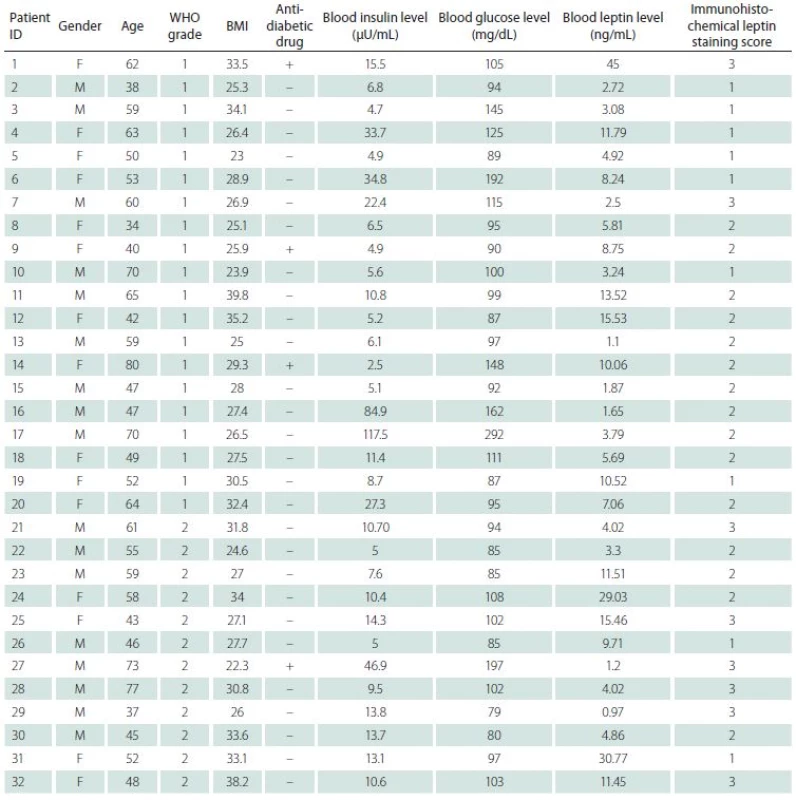
Soubor a metody: Do studie bylo zařazeno 20 pacientů s meningeomem stupně I a 12 pacientů s meningeomem stupně II podle klasifikace nádorů CNS dle WHO. Byly stanoveny hodnoty glykemie nalačno, inzulinu v krvi a leptinu v krvi. Imunohistochemicky bylo stanoveno skóre barvení leptinu z parafinových bloků pacientů s meningeomem. Také byly zaznamenány hodnoty body mass indexu a antidiabetická léčba.
Výsledky: Nebyl zjištěn žádný statisticky významný vztah mezi skupinou s meningeomem stupně I a skupinou s meningeomem stupně II ve všech zkoumaných parametrech (body mass index, glykemie, hladina leptinu v krvi, skóre zbarvení leptinu). Užívání antidiabetik bylo mezi skupinami homogenní.
Závěr: Tato studie neposkytla důkazy o možné souvislosti mezi leptinem a tvorbou intrakraniálního meningeomu. Pro potvrzení takového vztahu je však nutný výzkum s větším rozsahem skupin pacientů, včetně meningeomů stupně III.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Klíčová slova:
leptin – leptinový receptor – meningeóm
Authors:
F. Sahinturk; E. Sonmez; P. Borcek; N. Altinors
Authors place of work:
Faculty of Medicine, Başkent University, Turkey
Published in the journal:
Cesk Slov Neurol N 2019; 82(2): 166-170
Category:
Původní práce
doi:
https://doi.org/10.14735/amcsnn2019csnn.eu1
Summary
Introduction: Meningiomas are the most frequent benign tumours of the intracranial cavity accounting for around 30% of all intracranial tumours. The majority of meningiomas are actually benign while a certain subset demonstrate a higher incidence of recurrence and unfavourable morbidity and mortality rates. Leptin, the product of the obese (ob) gene, is a 16-kDA polypeptide which is located on human chromosome 7. It plays a crucial role in the regulation of body weight by controlling food intake, energy metabolism and neuroendocrine function.
Aim: To search for a possible relationship between leptin and intracranial meningioma formation. A prospective control clinical study was used.
Patients and methods: According to WHO classification of CNS tumours, 20 patients with grade I and 12 patients harbouring grade II meningiomas were included in the study. Fasting blood glucose, blood insulin and leptin levels were determined. Leptin staining scores were evaluated immunohistochemically from the parafin blocks of the meningioma patients. Body mass index values and antidiabetic drug treatment were also noted.
Results: No statistically significant relationship was noted between the grade I and grade II meningioma groups in all the parameters searched (body mass index, blood glucose levels, blood leptin levels, leptin staining score). The use of antidiabetic drug treatment was homogenous between the groups.
Conclusion: The present study did not provide any evidence about a possible association between leptin and intracranial meningioma formation. However, research with a larger volume of patient groups, including grade III meningiomas is needed in order to substantiate such a relationship.
瘦素在颅内脑膜瘤的发生中起作用吗?
作品简介:
脑膜瘤是颅内最常见的良性肿瘤,约占颅内肿瘤的30%。大多数脑膜瘤实际上是良性的,而有一部分脑膜瘤的复发率较高,发病率和死亡率较低。瘦素是肥胖(ob)基因的产物,是一种位于人类7号染色体上的16-kDA多肽。它通过控制食物摄入量、能量代谢和神经内分泌功能,在调节体重方面发挥着至关重要的作用。
目的:
为了寻找瘦素与颅内脑膜瘤形成之间可能的关系,采用了前瞻性对照临床研究。
患者和方法:
根据WHO对中枢神经系统肿瘤的分类,本研究纳入20例I级脑膜瘤患者和12例II级脑膜瘤患者。测定空腹血糖、血胰岛素和瘦素水平。采用免疫组化方法对脑膜瘤石蜡切片进行瘦素染色评分。体重指数和抗糖尿病药物治疗也被注意。
结果:
检索到的所有参数(体重指数、血糖水平、血清瘦素水平、瘦素染色评分),I级脑膜瘤组与II级脑膜瘤组间无统计学意义相关。两组间抗糖尿病药物治疗的使用情况相同。
结论:
本研究未提供瘦素与颅内脑膜瘤形成可能相关的任何证据。然而,需要对包括III级脑膜瘤在内的大量患者群体进行研究,以证实这种关系。
关键词:
瘦素受体脑膜瘤
Keywords:
meningeoma – leptin – leptin receptor
Introductıon
Meningiomas are the most frequent benign tumours of the intracranial cavity accounting for around 30% of all intracranial tumours [1]. The majority of meningiomas are actually benign while a certain subset demonstrate a higher incidence of recurrence and unfavourable morbidity and mortality rates. The first choice of treatment is still surgery with the aim of total resection. Radiosurgery is generally adjuvant mode of treatment for subtotally resected, atypical and malignant forms. In some specific situations, radiosurgery may be the initial form of therapy.
Leptin, the product of the obese (ob) gene, is a 16-kDA polypeptide which is located on human chromosome 7. It plays a crucial role in the regulation of body weight by controlling food intake, energy metabolism and neuroendocrine function [2,3]. Leptin is mainly secreted from adipose tissue. It elicits its effects by interacting with specific receptors (ObR) at the level of hypothalamus. Leptin was thought to be produced only by the adipocytes in the early studies. However, stomach [4], heart, skeletal muscle [5], placenta [6] and mammary gland were also reported as being other sources in recent human studies. The presence of leptin and leptin receptors have been demonstrated in the brain and pituitary of rats [7,8]. After the results of these studies, it is not accurate to limit the effects of leptin as responsible for body weight control only. Leptin was also shown to regulate different physiological and pathological processes, including brain growth [9], reproduction, immunity function and cancer progression [2,10,11].
To our knowledge, this is the first study that evaluates the relationship between body mass index (BMI), blood leptin levels and leptin receptor expression in meningioma patients.
Patients and methods
Tissue samples
The patient group included 32 patients, age range between 34 and 80 years and average of 54.9 years. The male/ female ratio was 1.12/ 1 (17 males, 15 females). On the basis of WHO classification of CNS tumours, 20 patients had grade I and 12 patients had grade II meningiomas. Grade I patients formed group 1 while grade II patients constituted group 2.
Immunohistochemical staining
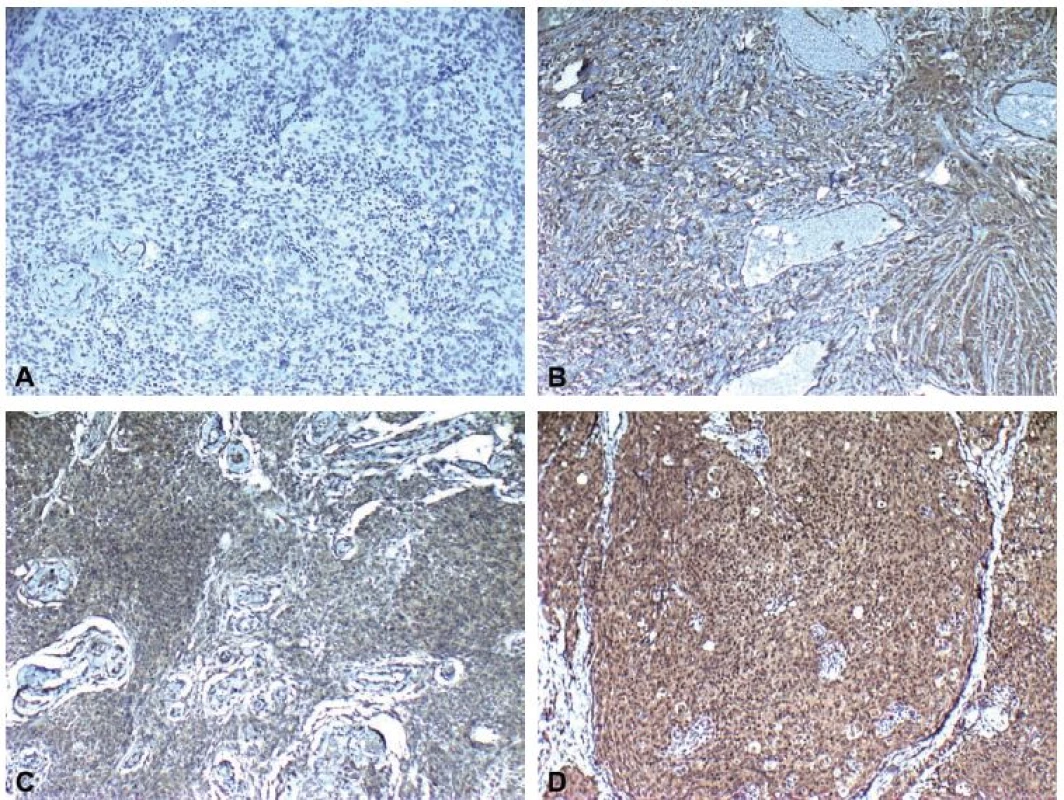
Haematoxylin and eosin sections from the tumour of each patient were reviewed and suitable paraffin blocks were selected for immunohistochemical staining. Four-µm sections were obtained, deparaffinized and an antigen retrieval procedure was carried out by microwaving the sections in ethylenediaminetetraacetic acid for 20 min. Staining with leptin antibody (rabbit polyclonal antibody, clone: orb10976, Biorbyt, San Francisco, CA, USA) was performed using avidin-streptavidin method. Positive and negative batch controls showed appropriate staining. The intensity of immunolabelling was scored as follows: 0 (no immunoreactivity), 1+ (weak immunoreactivity), 2+ (moderate immunoreactivity), 3+ (intense immunoreactivity).
Biochemical analysis
The fasting peripheral venous blood samples of 5 cc were obtained from each patient for glucose, insulin and leptin levels. They were centrifuged at 3,000 rpm for 10 min and stored at –20 °C for maximum of 3 months. The DIAsource Leptin-EASIA kit (DIAsource Immuno Assays S.A., Louvain-la-Neuve, Belgium) was used for serum leptin analysis. The results were obtained using ELISA-AID software (ElisaKit.com Pty Ltd, Scoresby, VIC, Australia). The BMI and use of antidiabetic drug s were noted.
Statistical analysis
All data collected throughout the clinical study were analysed using SPSS 15 statistical software (IBM Corp., Chicago, IL, USA). Non-parametric tests were used because of the patient numbers in the groups. Nonparametric groups were evaluated by the Mann-Whitney U test, and the chi-square and/ or Fisher’s exact tests were applied for intergroup comparisons. A p < 0.05 was considered statistically significant.
Results
There were 15 females and 17 males in the study. Nine male and 11 female patients formed group 1 while 8 male and 4 female patients were present in group 2. The patients’ age in the groups ranged from 34 to 80 years with an average age of 57.65 and 54.50, resp. In terms of age and gender, there was no statistically significant difference observed between the groups (p > 0.05) (Fig. 1).
Obr. 1. Sloupcový graf znázorňující výsledky studie leptinu. Nebyly nalezeny žádné signifikantní rozdíly mezi parametry. BMI – index tělesné hmotnosti; WHO – Světová zdravotnická organizace
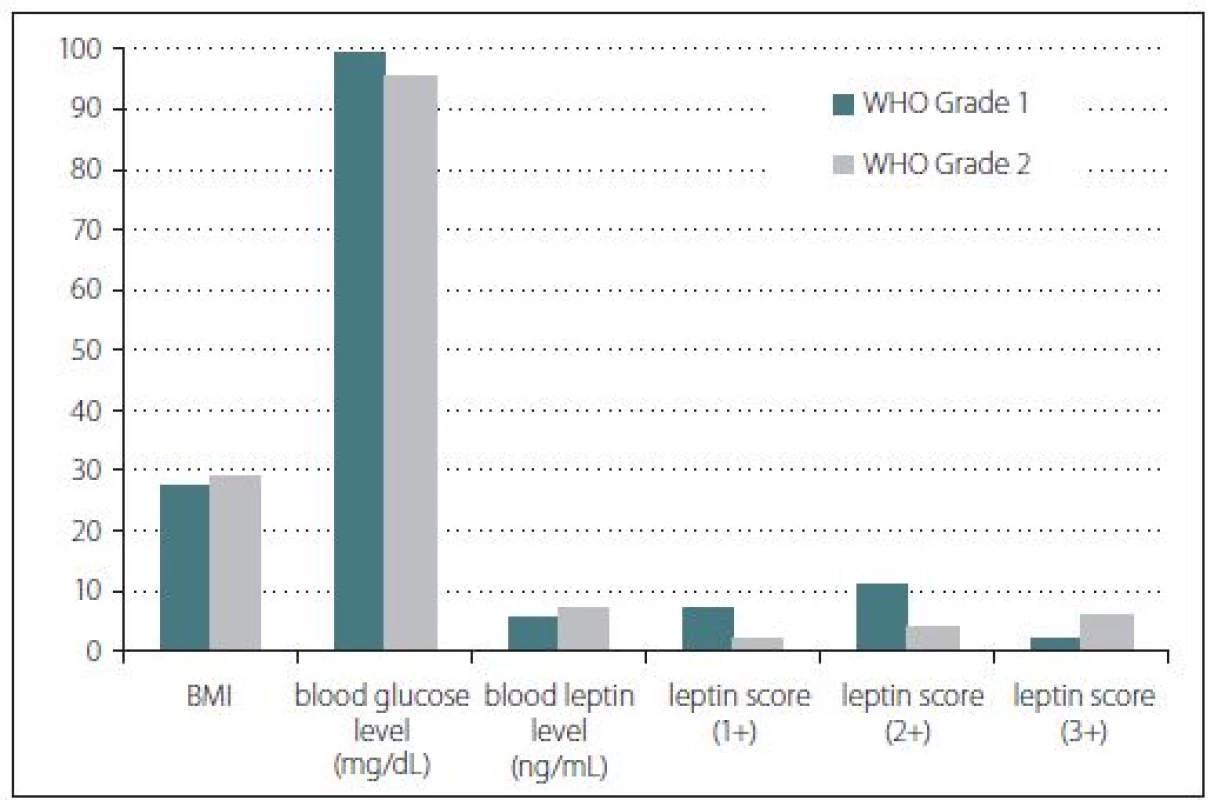
Body mass index, blood glucose and blood leptin levels were some of the other studied parameters. According to the BMI calculations, the median values were found as 27.45 for group 1 and 29.25 for group 2 patients. However, this difference was not statistically significant (p > 0.05). Furthermore, median blood glucose level of group 1 was found as 99.5 mg/ dl while group 2 had a median glucose level of 95.5 mg/ dl. This difference was not statistically significant either (p > 0.05). Serum median leptin levels for group 1 and group 2 were found as 5.75 and 7.28 ng/ ml, resp. However, these results did not show any statistically significant difference (p > 0.05) (Fig. 1). Three patients with a history of antidiabetic treatment were found in group 1 while only one such patient was present in group 2.
Immunohistochemical staining for leptin receptors demonstrated different staining scores in terms of group 1 and 2 (Fig. 2). However, this difference was not found to be statistically significant with a p = 0.054. The data of the study are shown in Tab. 1.
Obr. 2. Imunohistochemické skóre zabarvení ukazující A) žádné (0), B) slabé (1), C) střední (2) a D) intenzivní (3) imunoreaktivitu (leptin × 100).
Discussion
Meningiomas are believed to originate from the arachnoid cap cells. Different etiologic factors have been proposed in literature. Among these, trauma and ionising radiation are the well known ones [1,12–18]. Schneider et al. [19] retrospectively analysed the risk factors and predisposing factors for the development of symptomatic meningioma during adult life in a group of 306 patients. The results of the study revealed association of pre-existing diabetes with meningioma in the age group of 40–69 years. In female patients, significant association of arterial hypertension and meningioma formation was noted in the age group of 60–69 years. Rheumatoid arthritis had negative association in both sexes over 50 years of age. Bronchial asthma, smoking and obesity were found to be not significantly related with meningioma development. A secondary primary tumour was present in 12 cases, the most frequent tumour being breast cancer (5/ 12). There were 32 patients in our study. The patients’ age in the groups ranged from 34 to 80 years with an average age of 57.65 and 54.50, resp. In terms of age and gender, there was no statistically significant difference observed between the groups.
Body mass index is a strong suspect in the development of meningioma. There are several studies in the literature that refer to the relationship between BMI and meningioma. Aghi et al. [20] retrospectively studied 32 male patients with meningioma. The average BMI was 30.2 kg/ m2 and 47% of this group was found to be obese. They compared the postoperative complications of the meningioma group with those of the aneurysm and glioma group. The results showed high risk of postoperative complications in obese male patients harbouring meningioma. In a retrospective US study including 72,257 patients whounderwent surgery for intracranial meningioma between 1998 and 2007, the association of weight and outcomes of me-ningioma was evaluated. The authors con-cluded that weight loss was the single most critical factor in patients experiencing higher mortality, complications, hospital charges and longer hospital stay. Malignant tumours were more common in patients with weight loss (6.40 vs 4.30%, p = 0.03). Obesity seemed to reduce mortality (odds ratio [OR] 0.47; p = 0.0006) and complications (OR 0.8; p = 0.0007) in women. In The HUNT study, Wiedmann et al. [21] reported that BMI was positively associated with meningioma risk in women. Furthermore, in a recent meta analysis, Shao et al. [22] suggested that obesity, but not being overweight, was associated with an increased risk of meningioma. The results of a European prospective cohort, including 203 meningioma patients, supported an increase in risk of meningioma with higher BMI among both men and women [23]. In our study, only 4 patients were in normal ranges while 12 patients were obese and 16 patients were overweight according to the BMI calculations. When we compared the normal BMI patients and the overweight plus obese patients, this difference was statistically significant (p < 0.05). However, median values for BMI were found as 27.45 for group 1 and 29.25 for group 2. This difference was not statistically significant.
Leptin and leptin receptors were shown to be overexpressed in various tumours, including breast, colorectal, endometrial and prostate. But the potential role of leptin in human brain tumours has not been explored in detail yet. Most leptin studies on human brain tumours focused on malignant tumours, especially glioblastomas [24–27]. Knerr et al. [28] assessed human intracranial tumours for their gene expression pattern of leptin and leptin receptors. They investigated 35 inactive pituitary adenomas, 8 meningiomas, 7 prolactinomas, 7 somatotropinomas and 7 astrocytomas. They concluded that leptin and its receptors were expressed in various intracranial tumours but their distribution did not exhibit a characteristic or functional pattern. Riolfi et al. [29] were the first to demonstrate leptin and receptor expression on the protein level in a large series of brain cancer specimens. They have screened 87 human brain tumour biopsies using immunohistochemistry and have detected leptin and ObR in 55.20% and 60.90% of cases, resp. Furthermore, the authors observed highly significant association between leptin/ ObR and the degree of tumour malignancy. The leptin/ ObR system was highly expressed in glioblastomas and anaplastic astrocytomas, whereas there was a lower expression of both markers noted in low-grade astrocytomas and gangliogliomas.
Maffei et al. [30] reported that obese humans have high circulating leptin levels that are directly correlated to the total amount of adipose tissue. In our study, median serum leptin values for overweight and obese patients were found to be 5.75 and 10.985 ng/ ml, resp. Statistically significant difference was observed between the overweight and obese patients. These results were similar to the study conducted by Maffei et al. Furthermore, serum median leptin levels for group 1 and group 2 were found as 5.75 and 7.28 ng/ ml, resp. But, these results did not show any statistically significant difference.
Riolfi et al. [29] reported that in addition to endogenous hormones, such as oestrogen or progesterone, or fatty tissue-associated proinflammatory cytokines, leptin receptor expression status might be a risk factor for meningioma growth and progression. They have screened 158 meningiomas (low-grade meningiomas, N = 114; high-grade meningiomas, N = 44). Conversely, our study is limited by the number of patients as well as having no grade III meningioma patients.
Riolfi et al. [29] also reported a positive correlation between the expression of leptin/ leptin receptor and the degree of tumour malignancy. Similarly in breast and gastric cancers, overexpression of leptin/ leptin receptor was significantly associated with greater tumour size, higher tumour grade/ stage and poorer prognosis. In our study, immunohistochemical staining for leptin receptors demonstrated different staining scores in terms of grade I and II meningiomas. However, this difference was not found to be statistically significant with a p = 0.054. We speculate that this borderline insignificant result with a 0.004 difference could originate from the limited number of group 2 patients.
To our knowledge, the main drawback of the present study is the limited number of patients and having no grade III meningioma patients due to lack of sufficient patient cooperation and participation.
Conclusion
Our data reveal that leptin receptors are found in various numbers in meningioma patients and may be correlated with tumour grade. Further studies with larger cohorts are needed to reveal an association between leptin and intracranial meningioma development.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 24. 8. 2018
Accepted for print: 3. 1. 2019
Fikret Sahinturk, MD
Faculty of Medicine
Başkent University
Bağlıca Kampüsü
Eskişehir Yolu 20. Km.
06810 Etimesgut/Ankara
Turkey
e-mail: fikretsahinturk@gmail.com
Zdroje
1. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol 2010; 99(3): 307–314. doi: 10.1007/ s11060-010-0386-3.
2. Ahima RS, Saper CB, Flier JS et al. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 2000; 21(3): 263–307. doi: 10.1006/ frne.2000.0197.
3. Morrison CD. Leptin signaling in brain: a link between nutrition and cognition? Biochim Biophys Acta 2009; 1792(5): 401–408. doi: 10.1016/ j.bbadis.2008.12.004.
4. Bado A, Levasseur S, Attoub S et al. The stomach is a source of leptin. Nature 1998; 394(6695): 790–793. doi: 10.1038/ 29547.
5. Wang J, Liu R, Hawkins M et al. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998; 393(6686): 684–688. doi: 10.1038/ 31474.
6. Hoggard N, Hunter L, Duncan JS et al. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci U S A 1997; 94(20): 11073–11078.
7. Elmquist JK, Bjorbaek C, Ahima RS et al. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 1998; 395(4): 535–547.
8. Morash BA, Imran A, Wilkinson D et al. Leptin receptors are developmentally regulated in rat pituitary and hypothalamus. Mol Cell Endocrinol 2003; 210(1–2): 1–8.
9. Steppan CM and Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun 1999; 256(3): 600–602. doi: 10.1006/ bbrc.1999.0382.
10. Clement K, Vaisse C, Lahlou N et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998; 392(6674): 398–401. doi: 10.1038/ 32911.
11. Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev 1998; 56(Pt 2): s38–s46.
12. Aras Y, Akcakaya MO, Aydoseli A et al. Multiple atypical recurrent meningiomas 13 years after radiotherapy for unilateral retinoblastoma: case report and review of the literature. Neurol Neurochir Pol 2013; 47(1): 80–85.
13. Artico M, Cervoni L, Carloia S et al. Development of intracranial meningiomas at the site of cranial fractures. Remarks on 15 cases. Acta Neurochir (Wien) 1995; 136(3–4): 132–134.
14. Cervoni L, Celli P, Maraglino C et al. Intracranial meningioma at the site of a previous cranial fracture: case report and review of the literature. Ital J Neurol Sci 1996; 17(1): 79–81.
15. Chourmouzi D, Papadopoulou E, Kontopoulos A et al. Radiation-induced intracranial meningioma and multiple cavernomas. BMJ Case Rep 2013; 2013: pii: bcr2013010041. doi: 10.1136/ bcr-2013-010041.
16. Francois P, N‘Dri D, Bergemer-Fouquet AM et al. Post-traumatic meningioma: three case reports of this rare condition and a review of the literature. Acta Neurochir (Wien) 2010; 152(10): 1755–1760. doi: 10.1007/ s00701-010-0730-6.
17. Salvati M, Cervoni L, Artico M. High-dose radiation-induced meningiomas following acute lymphoblastic leukemia in children. Childs Nerv Syst 1996; 12(5): 266–269.
18. Vinchon M, Leblond P, Caron S et al. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Childs Nerv Syst 2011; 27(3): 445–453. doi: 10.1007/ s00381-011-1390-4.
19. Schneider B, Pulhorn H, Rohrig B et al. Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer Detect Prev 2005; 29(5): 440–447. doi: 10.1016/ j.cdp.2005.07.002.
20. Aghi MK, Eskandar EN, Carter BS et al. Increased prevalence of obesity and obesity-related postoperative complications in male patients with meningiomas. Neurosurgery 2007; 61(4): 754–760. doi: 10.1227/ 01.NEU.0000298903.63635.E3.
21. Wiedmann M, Brunborg C, Lindemann K et al. Body mass index and the risk of meningioma, glioma and schwannoma in a large prospective cohort study (The HUNT Study). Br J Cancer 2013; 109(1): 289–294. doi: 10.1038/ bjc.2013.304.
22. Shao C, Bai LP, Qi ZY et al. Overweight, obesity and meningioma risk: a meta-analysis. PLoS One 2014; 9(2): e90167. doi: 10.1371/ journal.pone.0090167.
23. Michaud DS, Bove G, Gallo V et al. Anthropometric measures, physical activity, and risk of glioma and meningioma in a large prospective cohort study. Cancer Prev Res (Phila) 2011; 4(9): 1385–1392. doi: 10.1158/ 1940-6207.CAPR-11-0014.
24. Ferla R, Bonomi M, Otvos L Jr et al. Glioblastoma-derived leptin induces tube formation and growth of endothelial cells: comparison with VEGF effects. BMC Cancer 2011; 11: 303. doi: 10.1186/ 1471-2407-11-303.
25. Ferla R, Haspinger E, Surmacz E. Metformin inhibits leptin-induced growth and migration of glioblastoma cells. Oncol Lett 2012; 4(5): 1077–1081. doi: 10.3892/ ol.2012.843.
26. Han G, Wang L, Zhao R et al. Leptin promotes human glioblastoma growth through activating signal transducers and activators of transcription 3 signaling. Brain Res Bull 2012; 87(2–3): 274–279. doi: 10.1016/ j.brainresbull.2011.11.007.
27. Lawrence JE, Cook NJ, Rovin RA et al. Leptin promotes glioblastoma. Neurol Res Int 2012; 2012: 870807. doi: 10.1155/ 2012/ 870807.
28. Knerr I, Schuster S, Nomikos P et al. Gene expression of adrenomedullin, leptin, their receptors and neuropeptide Y in hormone-secreting and non-fuctioning pituitary adenomas, meningiomas and malignant intracranial tumours in humans. Neuropathol Appl Neurobiol 2001; 27(3): 215–222.
29. Riolfi M, Ferla R, Del Valle L et al. Leptin and its receptor are overexpressed in brain tumors and correlate with the degree of malignancy. Brain Pathol 2010; 20(2): 481–489. doi: 10.1111/ j.1750-3639.2009.00323.x.
30. Maffei M, Halaas J, Ravussin E et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995; 1(11): 1155–1161.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2019 Číslo 2
Nejčtenější v tomto čísle
- Intradural extramedullary spinal cord tumors
- Rapid diagnostics of chemokine CXCL13 in the cerebrospinal fluid of patients with neuroborreliosis
- Genetics of neuromuscular diseases
- Multiple sclerosis and pregnancy from a gynecologist‘s perspective – as sisted reproduction options
